Class 12 Exam > Class 12 Questions > Directions: These questions consist of two s...
Start Learning for Free
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4
Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.
- a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.
- b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.
- c)If the Assertion is correct but Reason is incorrect.
- d)If both the Assertion and Reason are incorrect.
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Directions: These questions consist of two statements, each printed a...
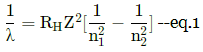
For the Lyman series, n1 = 1. For the next level, n2 = 2
so by eq.1 the ratio of minimum and maximum wavelength is 3/4.
Hence the assertion is correct.
Yes, the Lyman series constitutes spectral lines corresponding to the transition from higher energy to the ground state of the hydrogen atom. so the reason is also correct. But the reason is not the correct explanation of the assertion. Hence option 'B' is correct.
Most Upvoted Answer
Directions: These questions consist of two statements, each printed a...
Assertion: In Lyman series, the ratio of minimum and maximum wavelength is 3/4
Reason: Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.
Explanation:
The Lyman series is a series of spectral lines in the ultraviolet region of the electromagnetic spectrum that are emitted when electrons in hydrogen atoms transition from higher energy levels to the ground state.
Assertion: In Lyman series, the ratio of minimum and maximum wavelength is 3/4
This statement is correct. The Lyman series consists of a series of spectral lines that correspond to transitions from higher energy levels to the ground state of the hydrogen atom. The wavelengths of these spectral lines can be calculated using the Rydberg formula:
1/λ = R_H * (1/n_1^2 - 1/n_2^2)
where λ is the wavelength, R_H is the Rydberg constant for hydrogen, and n_1 and n_2 are the principal quantum numbers of the initial and final energy levels, respectively.
For the Lyman series, the initial energy level (n_1) is always equal to 1 (the ground state), and the final energy level (n_2) can be any value greater than 1. The minimum wavelength in the Lyman series corresponds to the transition from the first excited state (n_2 = 2) to the ground state (n_1 = 1). The maximum wavelength corresponds to the transition from the highest energy level (n_2 = infinity) to the ground state (n_1 = 1).
The ratio of the minimum and maximum wavelength can be calculated as:
(1/λ_min)/(1/λ_max) = (n_2^2 - n_1^2)/(n_2^2 - 1) = (2^2 - 1^2)/(2^2 - 1) = 3/4
Therefore, the ratio of the minimum and maximum wavelength in the Lyman series is indeed 3/4.
Reason: Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.
This statement is also correct. The Lyman series specifically corresponds to transitions from higher energy levels to the ground state of the hydrogen atom. Each spectral line in the Lyman series represents a specific transition between two energy levels, with the ground state (n=1) being the final energy level for all transitions in this series.
Therefore, both the Assertion and Reason are correct. However, the Reason is not a correct explanation of the Assertion. The Reason simply restates the definition of the Lyman series and does not provide an explanation for why the ratio of minimum and maximum wavelength is 3/4 in this series. The calculation based on the Rydberg formula provides the explanation for this ratio. Hence, the correct answer is option B.
Reason: Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.
Explanation:
The Lyman series is a series of spectral lines in the ultraviolet region of the electromagnetic spectrum that are emitted when electrons in hydrogen atoms transition from higher energy levels to the ground state.
Assertion: In Lyman series, the ratio of minimum and maximum wavelength is 3/4
This statement is correct. The Lyman series consists of a series of spectral lines that correspond to transitions from higher energy levels to the ground state of the hydrogen atom. The wavelengths of these spectral lines can be calculated using the Rydberg formula:
1/λ = R_H * (1/n_1^2 - 1/n_2^2)
where λ is the wavelength, R_H is the Rydberg constant for hydrogen, and n_1 and n_2 are the principal quantum numbers of the initial and final energy levels, respectively.
For the Lyman series, the initial energy level (n_1) is always equal to 1 (the ground state), and the final energy level (n_2) can be any value greater than 1. The minimum wavelength in the Lyman series corresponds to the transition from the first excited state (n_2 = 2) to the ground state (n_1 = 1). The maximum wavelength corresponds to the transition from the highest energy level (n_2 = infinity) to the ground state (n_1 = 1).
The ratio of the minimum and maximum wavelength can be calculated as:
(1/λ_min)/(1/λ_max) = (n_2^2 - n_1^2)/(n_2^2 - 1) = (2^2 - 1^2)/(2^2 - 1) = 3/4
Therefore, the ratio of the minimum and maximum wavelength in the Lyman series is indeed 3/4.
Reason: Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.
This statement is also correct. The Lyman series specifically corresponds to transitions from higher energy levels to the ground state of the hydrogen atom. Each spectral line in the Lyman series represents a specific transition between two energy levels, with the ground state (n=1) being the final energy level for all transitions in this series.
Therefore, both the Assertion and Reason are correct. However, the Reason is not a correct explanation of the Assertion. The Reason simply restates the definition of the Lyman series and does not provide an explanation for why the ratio of minimum and maximum wavelength is 3/4 in this series. The calculation based on the Rydberg formula provides the explanation for this ratio. Hence, the correct answer is option B.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer?
Question Description
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer?.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer?.
Solutions for Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.Assertion : In Lyman series, the ratio of minimum and maximum wavelength is 3/4Reason : Lyman series constitute spectral lines corresponding to transition from higher energy to ground state of the hydrogen atom.a)If both Assertion and Reason are correct and the Reason is a correct explanation of the Assertion.b)If both Assertion and Reason are correct but Reason is not a correct explanation of the Assertion.c)If the Assertion is correct but Reason is incorrect.d)If both the Assertion and Reason are incorrect.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















