Class 11 Exam > Class 11 Questions > How to find oxidation number ?
Start Learning for Free
How to find oxidation number ?
Verified Answer
How to find oxidation number ?
In chemistry, the terms "oxidation" and "reduction" refer to reactions in which an atom (or group of atoms) loses or gains electrons, respectively. Oxidation numbers are numbers assigned to atoms (or groups of atoms) that help chemists keep track of how many electrons are available for transfer and whether given reactants are oxidized or reduced in a reaction. The process of assigning oxidation numbers to atoms can range from remarkably simple to somewhat complex, based on the charge of the atoms and the chemical composition of the molecules they are a part of. To complicate matters, some elements can have more than one oxidation number. Luckily, the assignment of oxidation numbers is governed by well-defined, easy-to follow rules, though knowledge of basic chemistry and algebra will make navigation of these rules much easier.
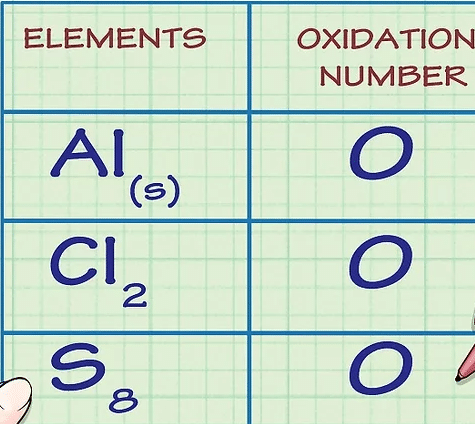
Determine whether the substance in question is elemental. Free, uncombined elemental atoms always have an oxidation number of 0. This is true both for atoms whose elemental form is composed of a lone atom, as well as atoms whose elemental form is diatomic or polyatomic.
For example, Al(s) and Cl2 both have oxidation numbers of 0 because they are in their uncombined elemental forms.
Note that sulfur's elemental form, S8, or octasulfur, though irregular, also has an oxidation number of 0.
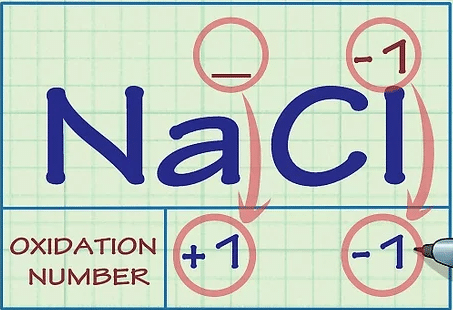
Determine whether the substance in question is an ion. Ions have oxidation numbers equal to their charge. This is true both for ions that are not bound to any other elements as well as for ions that form part of an ionic compound.
For instance, the ion Cl- has an oxidation number of -1.
The Cl ion still has an oxidation number of -1 when it's part of the compound NaCl. Because the Na+ ion, by definition, has a charge of +1, we know that the Cl- ion has a charge of -1, so its oxidation number is still -1.
 This question is part of UPSC exam. View all Class 11 courses
This question is part of UPSC exam. View all Class 11 courses
Most Upvoted Answer
How to find oxidation number ?
The oxidation number of a free element is always 0.
The oxidation number of a monatomic ion equals the charge of the ion.
The oxidation number of H is +1, but it is -1 in when combined with less electronegative elements.
The oxidation number of O in compounds is usually -2, but it is -1 in peroxides.
The oxidation number of a Group 1 element in a compound is +1.
The oxidation number of a Group 2 element in a compound is +2.
The oxidation number of a Group 17 element in a binary compound is -1.
The sum of the oxidation numbers of all of the atoms in a neutral compound is 0.
The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion.
EXAMPLE:
What is the oxidation number of Cr in CrCl3 ?
Solution:
We use what rules we can to determine the oxidation numbers.
Rule 7 states that the oxidation number of Cl is -1.
We write the oxidation number of the element above its symbol and the total for 3 Cl atoms below the symbol.
This gives Cr-1Cl3
mll-3mm.
Rule 8 states the numbers along the bottom must add up to zero. So the number under Cr must be +3.
This gives Cr-1Cl3
m+3ll-3mm.
There is only one Cr atom, so its oxidation number is +3.
This gives +3Cr-1Cl3
mmmmm+3ll-3mm.
The oxidation number of Cr in CrCl3 is +3.
Here is a chart showing the oxidation numbers of the atoms in some common elements and compounds.
The oxidation number of a monatomic ion equals the charge of the ion.
The oxidation number of H is +1, but it is -1 in when combined with less electronegative elements.
The oxidation number of O in compounds is usually -2, but it is -1 in peroxides.
The oxidation number of a Group 1 element in a compound is +1.
The oxidation number of a Group 2 element in a compound is +2.
The oxidation number of a Group 17 element in a binary compound is -1.
The sum of the oxidation numbers of all of the atoms in a neutral compound is 0.
The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion.
EXAMPLE:
What is the oxidation number of Cr in CrCl3 ?
Solution:
We use what rules we can to determine the oxidation numbers.
Rule 7 states that the oxidation number of Cl is -1.
We write the oxidation number of the element above its symbol and the total for 3 Cl atoms below the symbol.
This gives Cr-1Cl3
mll-3mm.
Rule 8 states the numbers along the bottom must add up to zero. So the number under Cr must be +3.
This gives Cr-1Cl3
m+3ll-3mm.
There is only one Cr atom, so its oxidation number is +3.
This gives +3Cr-1Cl3
mmmmm+3ll-3mm.
The oxidation number of Cr in CrCl3 is +3.
Here is a chart showing the oxidation numbers of the atoms in some common elements and compounds.
Community Answer
How to find oxidation number ?
The oxidation number of a free element is always 0.
The oxidation number of a monatomic ion equals the charge of the ion.
The oxidation number of H is +1, but it is -1 in when combined with less electronegative elements.
The oxidation number of O in compounds is usually -2, but it is -1 in peroxides.
The oxidation number of a monatomic ion equals the charge of the ion.
The oxidation number of H is +1, but it is -1 in when combined with less electronegative elements.
The oxidation number of O in compounds is usually -2, but it is -1 in peroxides.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
How to find oxidation number ?
Question Description
How to find oxidation number ? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about How to find oxidation number ? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How to find oxidation number ?.
How to find oxidation number ? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about How to find oxidation number ? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How to find oxidation number ?.
Solutions for How to find oxidation number ? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of How to find oxidation number ? defined & explained in the simplest way possible. Besides giving the explanation of
How to find oxidation number ?, a detailed solution for How to find oxidation number ? has been provided alongside types of How to find oxidation number ? theory, EduRev gives you an
ample number of questions to practice How to find oxidation number ? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























