JEE Exam > JEE Questions > Disaccharides are carbohydrates those contai...
Start Learning for Free
Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).
Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :
- a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketone
- b)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkage
- c)Surrose solution do not exhibit Mutarotation
- d)None of the above describes above abnormal behaviour
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Disaccharides are carbohydrates those contain two monosaccharides mol...
Disaccharides and their formation:
- Disaccharides are carbohydrates composed of two monosaccharide molecules joined together.
- Each monosaccharide molecule is in the hemiacetal form, which means it has an aldehyde or ketone group and a hydroxyl group.
- The two monosaccharides are joined by the elimination of a water molecule between two hydroxyl groups.
Dehydration and formation of acetal and glycoside:
- Dehydration involves the anomeric carbon of one monosaccharide.
- The hemiacetal hydroxyl group on the anomeric carbon is involved in the dehydration process.
- The resulting product is an acetal or a glycoside.
- In common language, it is called an acetal, while in the context of carbohydrates, it is called a glycoside.
Sucrose as a non-reducing sugar:
- Sucrose is a disaccharide composed of glucose and fructose molecules.
- Glucose has an aldehyde group and fructose has a ketone group.
- Aldehydes are more reactive than ketones.
- In sucrose, the aldehyde group of the glucose molecule is involved in forming a glycosidic linkage with the hydroxyl group of the fructose molecule.
- This glycosidic linkage blocks the free aldehydic or ketonic group in both glucose and fructose.
- As a result, sucrose does not have any free aldehydic or ketonic group, which is necessary for a sugar to exhibit reducing properties.
- Therefore, sucrose is a non-reducing sugar.
- Its hydrolysis products, glucose and fructose, are reducing sugars because they have free aldehyde and ketone groups.
Correct answer explanation:
- The correct answer is option 'B', which states that sucrose is a non-reducing sugar because it does not have a free aldehydic or ketonic group.
- This is true because the aldehyde group of the glucose component is involved in forming a glycosidic linkage, blocking the free aldehydic or ketonic group in both glucose and fructose.
- The other options are incorrect because they do not accurately describe the reason for sucrose being a non-reducing sugar.
- Option 'A' incorrectly states that glucose has an aldehyde group and fructose has a ketone group, but aldehyde is more reactive than ketone. While this is true, it is not the reason for sucrose being a non-reducing sugar.
- Option 'C' incorrectly states that sucrose solution does not exhibit mutarotation. Mutarotation refers to the spontaneous interconversion between different anomeric forms of a sugar. While sucrose does not exhibit mutarotation, it is not the reason for it being a non-reducing sugar.
- Option 'D' incorrectly states that none of the above describes the abnormal behavior of sucrose. However, the correct answer option 'B' accurately describes the reason for sucrose being a non-reducing sugar.
- Disaccharides are carbohydrates composed of two monosaccharide molecules joined together.
- Each monosaccharide molecule is in the hemiacetal form, which means it has an aldehyde or ketone group and a hydroxyl group.
- The two monosaccharides are joined by the elimination of a water molecule between two hydroxyl groups.
Dehydration and formation of acetal and glycoside:
- Dehydration involves the anomeric carbon of one monosaccharide.
- The hemiacetal hydroxyl group on the anomeric carbon is involved in the dehydration process.
- The resulting product is an acetal or a glycoside.
- In common language, it is called an acetal, while in the context of carbohydrates, it is called a glycoside.
Sucrose as a non-reducing sugar:
- Sucrose is a disaccharide composed of glucose and fructose molecules.
- Glucose has an aldehyde group and fructose has a ketone group.
- Aldehydes are more reactive than ketones.
- In sucrose, the aldehyde group of the glucose molecule is involved in forming a glycosidic linkage with the hydroxyl group of the fructose molecule.
- This glycosidic linkage blocks the free aldehydic or ketonic group in both glucose and fructose.
- As a result, sucrose does not have any free aldehydic or ketonic group, which is necessary for a sugar to exhibit reducing properties.
- Therefore, sucrose is a non-reducing sugar.
- Its hydrolysis products, glucose and fructose, are reducing sugars because they have free aldehyde and ketone groups.
Correct answer explanation:
- The correct answer is option 'B', which states that sucrose is a non-reducing sugar because it does not have a free aldehydic or ketonic group.
- This is true because the aldehyde group of the glucose component is involved in forming a glycosidic linkage, blocking the free aldehydic or ketonic group in both glucose and fructose.
- The other options are incorrect because they do not accurately describe the reason for sucrose being a non-reducing sugar.
- Option 'A' incorrectly states that glucose has an aldehyde group and fructose has a ketone group, but aldehyde is more reactive than ketone. While this is true, it is not the reason for sucrose being a non-reducing sugar.
- Option 'C' incorrectly states that sucrose solution does not exhibit mutarotation. Mutarotation refers to the spontaneous interconversion between different anomeric forms of a sugar. While sucrose does not exhibit mutarotation, it is not the reason for it being a non-reducing sugar.
- Option 'D' incorrectly states that none of the above describes the abnormal behavior of sucrose. However, the correct answer option 'B' accurately describes the reason for sucrose being a non-reducing sugar.
Free Test
FREE
| Start Free Test |
Community Answer
Disaccharides are carbohydrates those contain two monosaccharides mol...
Sucrose is having structure as
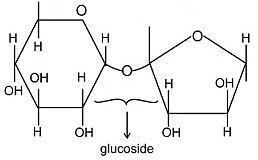
In above structure there is no free carbonyl centre of an aldehydic group present ; It is a non-reducing sugar.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer?
Question Description
Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer?.
Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Disaccharides are carbohydrates those contain two monosaccharides molecules, each in the hemiacetal form, joined together by the elimination of a water between two hydroxyl groups. Dehydration involves the anomeric carbon of one monosaccharide and may or may not involve the anomeric carbon of the other monosaccharide when the hemiacetal hydroxyl group on an anomeric carbon is involved in a dehydration, the resulting product is an acetal (in common) and glycoside (in carbohydrate).Sucrose is a non-reducing sugar (while its hydrolysis products glucose and fructose are reducing sugars) because :a)Glucose has aldehyde and fructose has ketone group and aldehyde is reactive than ketoneb)It has no free aldehydic or ketonic group. Aldehyde group of glucose component is marked as a glucoside linkagec)Surrose solution do not exhibit Mutarotationd)None of the above describes above abnormal behaviourCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























