JEE Exam > JEE Questions > Given below are two statements:Statement-I: S...
Start Learning for Free
Given below are two statements:
Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.
Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.
Choose the correct answer from the options given below.
Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.
Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.
Choose the correct answer from the options given below.
- a)Both Statement-I and Statement-II are true.
- b)Both Statement-I and Statement-II are false.
- c)Statement-I is true but Statement-II is false.
- d)Statement-I is false but Statement-II is true.
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Given below are two statements:Statement-I: Susceptibilities of parama...
Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.
The statement-I is true. The susceptibility of paramagnetic and ferromagnetic substances indeed increases with a decrease in temperature.
Explanation:
Paramagnetism:
- Paramagnetic materials are those materials that have unpaired electrons in their atoms or molecules.
- In the presence of an external magnetic field, these unpaired electrons align themselves with the field, resulting in a net magnetization of the material.
- The alignment of these magnetic moments is not perfect, and their orientations are random in the absence of a magnetic field.
- When a magnetic field is applied, the magnetic moments tend to align with the field, but due to thermal motion, they also experience randomizing effects.
- As the temperature decreases, the thermal motion of the electrons decreases, and they can align more easily with the external magnetic field.
- Therefore, the susceptibility (a measure of the material's response to an external magnetic field) of paramagnetic materials increases with a decrease in temperature.
Ferromagnetism:
- Ferromagnetic materials are those materials that have spontaneous magnetization even in the absence of an external magnetic field.
- In these materials, the magnetic moments of neighboring atoms or molecules align parallel to each other, creating magnetic domains.
- Below a certain temperature called the Curie temperature, these magnetic domains can align in a preferred direction, resulting in a strong net magnetization of the material.
- As the temperature decreases below the Curie temperature, the thermal motion of the atoms or molecules decreases, allowing for better alignment of the magnetic domains.
- This alignment leads to an increase in the susceptibility of the ferromagnetic material with decreasing temperature.
Therefore, both paramagnetic and ferromagnetic substances show an increase in susceptibility with a decrease in temperature.
Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.
The statement-II is true. Diamagnetism is indeed a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.
Explanation:
- Diamagnetic materials are those materials that have all their electrons paired and no net magnetic moment in the absence of an external magnetic field.
- When a magnetic field is applied, the motion of the electrons in their atomic or molecular orbitals induces a small magnetic moment in the opposite direction to the applied field.
- This induced magnetic moment opposes the applied field and causes the material to be weakly repelled by the magnetic field.
- The orbital motion of the electrons is responsible for the development of these induced magnetic moments.
- Diamagnetism is a universal property of all materials to some extent, but it is usually overshadowed by the stronger paramagnetic or ferromagnetic effects in most materials.
- In contrast to paramagnetic and ferromagnetic materials, the susceptibility of diamagnetic materials decreases with a decrease in temperature.
- This is because as the temperature decreases, the thermal motion of the electrons decreases, leading to a decreased induced magnetic moment in the opposite direction to the applied field.
Hence, Statement-II is also true.
Therefore, the correct answer is option 'A' - Both Statement-I and Statement-II are true.
The statement-I is true. The susceptibility of paramagnetic and ferromagnetic substances indeed increases with a decrease in temperature.
Explanation:
Paramagnetism:
- Paramagnetic materials are those materials that have unpaired electrons in their atoms or molecules.
- In the presence of an external magnetic field, these unpaired electrons align themselves with the field, resulting in a net magnetization of the material.
- The alignment of these magnetic moments is not perfect, and their orientations are random in the absence of a magnetic field.
- When a magnetic field is applied, the magnetic moments tend to align with the field, but due to thermal motion, they also experience randomizing effects.
- As the temperature decreases, the thermal motion of the electrons decreases, and they can align more easily with the external magnetic field.
- Therefore, the susceptibility (a measure of the material's response to an external magnetic field) of paramagnetic materials increases with a decrease in temperature.
Ferromagnetism:
- Ferromagnetic materials are those materials that have spontaneous magnetization even in the absence of an external magnetic field.
- In these materials, the magnetic moments of neighboring atoms or molecules align parallel to each other, creating magnetic domains.
- Below a certain temperature called the Curie temperature, these magnetic domains can align in a preferred direction, resulting in a strong net magnetization of the material.
- As the temperature decreases below the Curie temperature, the thermal motion of the atoms or molecules decreases, allowing for better alignment of the magnetic domains.
- This alignment leads to an increase in the susceptibility of the ferromagnetic material with decreasing temperature.
Therefore, both paramagnetic and ferromagnetic substances show an increase in susceptibility with a decrease in temperature.
Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.
The statement-II is true. Diamagnetism is indeed a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.
Explanation:
- Diamagnetic materials are those materials that have all their electrons paired and no net magnetic moment in the absence of an external magnetic field.
- When a magnetic field is applied, the motion of the electrons in their atomic or molecular orbitals induces a small magnetic moment in the opposite direction to the applied field.
- This induced magnetic moment opposes the applied field and causes the material to be weakly repelled by the magnetic field.
- The orbital motion of the electrons is responsible for the development of these induced magnetic moments.
- Diamagnetism is a universal property of all materials to some extent, but it is usually overshadowed by the stronger paramagnetic or ferromagnetic effects in most materials.
- In contrast to paramagnetic and ferromagnetic materials, the susceptibility of diamagnetic materials decreases with a decrease in temperature.
- This is because as the temperature decreases, the thermal motion of the electrons decreases, leading to a decreased induced magnetic moment in the opposite direction to the applied field.
Hence, Statement-II is also true.
Therefore, the correct answer is option 'A' - Both Statement-I and Statement-II are true.
Free Test
FREE
| Start Free Test |
Community Answer
Given below are two statements:Statement-I: Susceptibilities of parama...
According to curies law, magnetic susceptibility is inversely proportional to temperature for a fixed value of external magnetic field i.e. x = C/T.
The same is applicable for ferromagnet & the relation is given as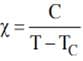 (TC is curie temperature) Diamagnetism is due to non-cooperative behaviour of orbiting electrons when exposed to external magnetic field.
(TC is curie temperature) Diamagnetism is due to non-cooperative behaviour of orbiting electrons when exposed to external magnetic field.
The same is applicable for ferromagnet & the relation is given as
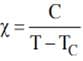 (TC is curie temperature) Diamagnetism is due to non-cooperative behaviour of orbiting electrons when exposed to external magnetic field.
(TC is curie temperature) Diamagnetism is due to non-cooperative behaviour of orbiting electrons when exposed to external magnetic field. Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer?
Question Description
Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer?.
Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer?.
Solutions for Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Given below are two statements:Statement-I: Susceptibilities of paramagnetic and ferromagnetic substances increase with decrease in temperature.Statement-II: Diamagnetism is a result of orbital motions of electrons developing magnetic moments opposite to the applied magnetic field.Choose the correct answer from the options given below.a)Both Statement-I and Statement-II are true.b)Both Statement-I and Statement-II are false.c)Statement-I is true but Statement-II is false.d)Statement-I is false but Statement-II is true.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























