JEE Exam > JEE Questions > For a first order reaction, the time required...
Start Learning for Free
For a first order reaction, the time required for completion of 90% reaction is 'x' times the half life of the reaction. The value of 'x' is (Given: In 10 = 2.303 and log 2 = 0.3010)
- a)1.12
- b)2.43
- c)3.32
- d)33.31
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
For a first order reaction, the time required for completion of 90% re...
First Order Reaction and Half Life
A first-order reaction is a chemical reaction that depends on the concentration of one reactant raised to the power of one, hence the name "first-order." A common example of a first-order reaction is the decay of radioactive isotopes. The rate of a first-order reaction is proportional to the concentration of the reactant. This means that as the concentration of the reactant decreases, the rate of the reaction also decreases.
The half-life of a reaction is the amount of time it takes for half of the reactant to be consumed. The half-life of a first-order reaction is defined by the equation:
t1/2 = (ln 2) / k
where t1/2 is the half-life, k is the rate constant, and ln is the natural logarithm.
90% Completion Time and Half Life
The question asks for the time required for completion of 90% reaction, which can be expressed as:
ln (Co/C) = k*t
where Co is the initial concentration, C is the concentration at time t, and k is the rate constant. Solving for t, we get:
t = (1/k) * ln (Co/C)
Now, we are told that this time is x times the half-life of the reaction. That is:
t = x * t1/2
Substituting the expression for t1/2 from above, we get:
x * (ln 2) / k = (1/k) * ln (Co/C)
Simplifying, we get:
ln (Co/C) = x * ln 2
Taking the antilog of both sides, we get:
Co/C = 2^x
Finally, solving for x, we get:
x = log2 (Co/C)
Using the given value of ln 10 and log 2, we get:
x = log10 (Co/C) / log10 2 = 3.32
Therefore, the value of x is 3.32.
A first-order reaction is a chemical reaction that depends on the concentration of one reactant raised to the power of one, hence the name "first-order." A common example of a first-order reaction is the decay of radioactive isotopes. The rate of a first-order reaction is proportional to the concentration of the reactant. This means that as the concentration of the reactant decreases, the rate of the reaction also decreases.
The half-life of a reaction is the amount of time it takes for half of the reactant to be consumed. The half-life of a first-order reaction is defined by the equation:
t1/2 = (ln 2) / k
where t1/2 is the half-life, k is the rate constant, and ln is the natural logarithm.
90% Completion Time and Half Life
The question asks for the time required for completion of 90% reaction, which can be expressed as:
ln (Co/C) = k*t
where Co is the initial concentration, C is the concentration at time t, and k is the rate constant. Solving for t, we get:
t = (1/k) * ln (Co/C)
Now, we are told that this time is x times the half-life of the reaction. That is:
t = x * t1/2
Substituting the expression for t1/2 from above, we get:
x * (ln 2) / k = (1/k) * ln (Co/C)
Simplifying, we get:
ln (Co/C) = x * ln 2
Taking the antilog of both sides, we get:
Co/C = 2^x
Finally, solving for x, we get:
x = log2 (Co/C)
Using the given value of ln 10 and log 2, we get:
x = log10 (Co/C) / log10 2 = 3.32
Therefore, the value of x is 3.32.
Free Test
FREE
| Start Free Test |
Community Answer
For a first order reaction, the time required for completion of 90% re...
A → Products
For a first order reaction,
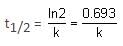
Time for 90% conversion,
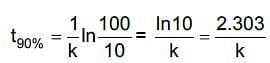
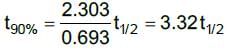
For a first order reaction,
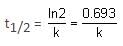
Time for 90% conversion,
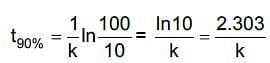
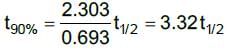
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer?
Question Description
For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer?.
For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer?.
Solutions for For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer?, a detailed solution for For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For a first order reaction, the time required for completion of 90% reaction is x times the half life of the reaction. The value of x is (Given: In 10 = 2.303 and log 2 = 0.3010)a)1.12b)2.43c)3.32d)33.31Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























