JEE Exam > JEE Questions > Ammonolysis of alkyl halides followed by the ...
Start Learning for Free
Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction is
- a)to remove basic impurities
- b)to activate NH3 used in the reaction
- c)to remove acidic impurities
- d)to increase the reactivity of alkyl halide
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Ammonolysis of alkyl halides followed by the treatment with NaOH solut...
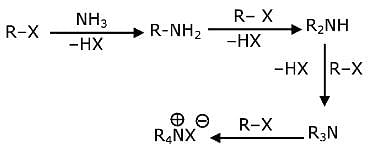
During the reaction, HX (acid) is formed.
So, NaOH is used to remove these acidic impurities.
Free Test
FREE
| Start Free Test |
Community Answer
Ammonolysis of alkyl halides followed by the treatment with NaOH solut...
Explanation:
The ammonolysis of alkyl halides involves the reaction of alkyl halides with ammonia (NH3) to form amines. This reaction can be carried out in the presence of a strong base like sodium hydroxide (NaOH) to improve the yield of amines.
Role of NaOH in the reaction:
The purpose of NaOH in the reaction is to remove acidic impurities that may be present in the reaction mixture. When alkyl halides are treated with ammonia, they can undergo hydrolysis to form the corresponding carboxylic acids. These carboxylic acids are acidic in nature and can hinder the formation of amines. Therefore, the presence of NaOH helps in neutralizing these acidic impurities and enhances the yield of amines.
Reaction mechanism:
The reaction proceeds through the following steps:
1. Ammonolysis of alkyl halide:
R-X + NH3 → R-NH2 + HX
In this step, the alkyl halide reacts with ammonia to form the corresponding primary amine and hydrogen halide. The primary amine is the desired product in this reaction.
2. Formation of carboxylic acid (acidic impurity):
R-X + H2O → R-COOH + HX
In the presence of water, the alkyl halide can undergo hydrolysis to form the corresponding carboxylic acid and hydrogen halide. This carboxylic acid is an acidic impurity that needs to be removed.
3. Neutralization of acidic impurities by NaOH:
R-COOH + NaOH → R-COONa + H2O
Sodium hydroxide reacts with the carboxylic acid to form the corresponding sodium carboxylate salt and water. This neutralization reaction helps in removing the acidic impurities from the reaction mixture.
Conclusion:
In conclusion, the purpose of NaOH in the ammonolysis of alkyl halides is to remove acidic impurities that may hinder the formation of amines. By neutralizing these acidic impurities, NaOH improves the yield of amines in the reaction.
The ammonolysis of alkyl halides involves the reaction of alkyl halides with ammonia (NH3) to form amines. This reaction can be carried out in the presence of a strong base like sodium hydroxide (NaOH) to improve the yield of amines.
Role of NaOH in the reaction:
The purpose of NaOH in the reaction is to remove acidic impurities that may be present in the reaction mixture. When alkyl halides are treated with ammonia, they can undergo hydrolysis to form the corresponding carboxylic acids. These carboxylic acids are acidic in nature and can hinder the formation of amines. Therefore, the presence of NaOH helps in neutralizing these acidic impurities and enhances the yield of amines.
Reaction mechanism:
The reaction proceeds through the following steps:
1. Ammonolysis of alkyl halide:
R-X + NH3 → R-NH2 + HX
In this step, the alkyl halide reacts with ammonia to form the corresponding primary amine and hydrogen halide. The primary amine is the desired product in this reaction.
2. Formation of carboxylic acid (acidic impurity):
R-X + H2O → R-COOH + HX
In the presence of water, the alkyl halide can undergo hydrolysis to form the corresponding carboxylic acid and hydrogen halide. This carboxylic acid is an acidic impurity that needs to be removed.
3. Neutralization of acidic impurities by NaOH:
R-COOH + NaOH → R-COONa + H2O
Sodium hydroxide reacts with the carboxylic acid to form the corresponding sodium carboxylate salt and water. This neutralization reaction helps in removing the acidic impurities from the reaction mixture.
Conclusion:
In conclusion, the purpose of NaOH in the ammonolysis of alkyl halides is to remove acidic impurities that may hinder the formation of amines. By neutralizing these acidic impurities, NaOH improves the yield of amines in the reaction.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer?
Question Description
Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer?.
Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Ammonolysis of alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction isa)to remove basic impuritiesb)to activate NH3 used in the reactionc)to remove acidic impuritiesd)to increase the reactivity of alkyl halideCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























