Civil Engineering (CE) Exam > Civil Engineering (CE) Questions > Which of the following minerals is formed by ...
Start Learning for Free
Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?
- a)Illite
- b)Albite
- c)Kaolinite
- d)Montmorillonite
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Which of the following minerals is formed by linking gibbsite sheets t...
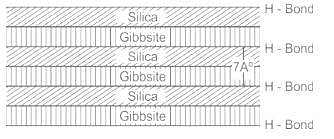
Kaolinite:
- The kaolinite structural unit is made up of gibbsite sheets(with aluminum atoms at their centers) joined to silica sheets through the unbalanced oxygen atoms at the apexes of the silicas.
- These basic units are then stacked one on top of the other to form a lattice of the mineral
- The successive 7 angstrom units are held together by hydrogen bonds.
- The strong bonding does not permit water to enter the lattice
- Thus, kaolinite minerals are stable and do not expand under saturation
- The total thickness of the structural unit of a Kaolinite mineral is 7 A°, where 1 A° = 10-10 meters.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following minerals is formed by linking gibbsite sheets t...
Mineral Formation: Kaolinite
Kaolinite is a mineral that is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica. Let's break down the process of kaolinite formation and understand it in detail.
1. Introduction to Kaolinite:
Kaolinite is a clay mineral that is commonly found in soils and sedimentary rocks. It is a layered mineral, meaning it is composed of stacked layers or sheets. These sheets are made up of silica tetrahedra and aluminum octahedra.
2. Structure of Kaolinite:
The structure of kaolinite consists of one layer of gibbsite (also known as aluminum hydroxide) sandwiched between two layers of silica. The gibbsite layer consists of aluminum octahedra linked together, while the silica layers consist of silica tetrahedra linked together. The layers are held together by weak hydrogen bonding.
3. Formation Process:
The formation of kaolinite involves the linking of gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica. This process can be explained in the following steps:
a) Weathering of Feldspar:
The weathering of feldspar minerals, such as orthoclase and plagioclase, is the initial step in the formation of kaolinite. Feldspar minerals are rich in aluminum and silica.
b) Dissolution and Hydrolysis:
During weathering, feldspar minerals undergo dissolution and hydrolysis reactions. The aluminum and silica ions are released from the feldspar minerals and become available for kaolinite formation.
c) Gibbsite Formation:
The released aluminum ions react with water and oxygen to form gibbsite. Gibbsite consists of aluminum hydroxide (Al(OH)3) units linked together to form a layer.
d) Silica Layer Formation:
The released silica ions react with water and oxygen to form silica units (SiO4) that link together to form a layer.
e) Linking of Layers:
The gibbsite layer is sandwiched between two silica layers. The unbalanced oxygen atoms at the top points of the silica layer provide binding sites for the gibbsite layer. The gibbsite layer attaches to the silica layers through weak hydrogen bonding and unbalanced oxygen atoms, resulting in the formation of kaolinite.
4. Significance of Kaolinite:
Kaolinite is an important mineral due to its various uses. It is extensively used in the paper, ceramics, paints, and rubber industries. It is also used in the production of porcelain and as a filler in pharmaceuticals. Additionally, kaolinite is used in agriculture as a soil conditioner and in the cosmetic industry.
In conclusion, kaolinite is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica. This process involves the weathering of feldspar minerals, dissolution and hydrolysis reactions, gibbsite formation, silica layer formation, and the linking of layers. Kaolinite has significant industrial and agricultural applications.
Kaolinite is a mineral that is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica. Let's break down the process of kaolinite formation and understand it in detail.
1. Introduction to Kaolinite:
Kaolinite is a clay mineral that is commonly found in soils and sedimentary rocks. It is a layered mineral, meaning it is composed of stacked layers or sheets. These sheets are made up of silica tetrahedra and aluminum octahedra.
2. Structure of Kaolinite:
The structure of kaolinite consists of one layer of gibbsite (also known as aluminum hydroxide) sandwiched between two layers of silica. The gibbsite layer consists of aluminum octahedra linked together, while the silica layers consist of silica tetrahedra linked together. The layers are held together by weak hydrogen bonding.
3. Formation Process:
The formation of kaolinite involves the linking of gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica. This process can be explained in the following steps:
a) Weathering of Feldspar:
The weathering of feldspar minerals, such as orthoclase and plagioclase, is the initial step in the formation of kaolinite. Feldspar minerals are rich in aluminum and silica.
b) Dissolution and Hydrolysis:
During weathering, feldspar minerals undergo dissolution and hydrolysis reactions. The aluminum and silica ions are released from the feldspar minerals and become available for kaolinite formation.
c) Gibbsite Formation:
The released aluminum ions react with water and oxygen to form gibbsite. Gibbsite consists of aluminum hydroxide (Al(OH)3) units linked together to form a layer.
d) Silica Layer Formation:
The released silica ions react with water and oxygen to form silica units (SiO4) that link together to form a layer.
e) Linking of Layers:
The gibbsite layer is sandwiched between two silica layers. The unbalanced oxygen atoms at the top points of the silica layer provide binding sites for the gibbsite layer. The gibbsite layer attaches to the silica layers through weak hydrogen bonding and unbalanced oxygen atoms, resulting in the formation of kaolinite.
4. Significance of Kaolinite:
Kaolinite is an important mineral due to its various uses. It is extensively used in the paper, ceramics, paints, and rubber industries. It is also used in the production of porcelain and as a filler in pharmaceuticals. Additionally, kaolinite is used in agriculture as a soil conditioner and in the cosmetic industry.
In conclusion, kaolinite is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica. This process involves the weathering of feldspar minerals, dissolution and hydrolysis reactions, gibbsite formation, silica layer formation, and the linking of layers. Kaolinite has significant industrial and agricultural applications.

|
Explore Courses for Civil Engineering (CE) exam
|

|
Similar Civil Engineering (CE) Doubts
Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer?.
Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Civil Engineering (CE).
Download more important topics, notes, lectures and mock test series for Civil Engineering (CE) Exam by signing up for free.
Here you can find the meaning of Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following minerals is formed by linking gibbsite sheets to sheets of silica through unbalanced oxygen atoms at the top points of silica?a)Illiteb)Albitec)Kaolinited)MontmorilloniteCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Civil Engineering (CE) tests.

|
Explore Courses for Civil Engineering (CE) exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























