JEE Exam > JEE Questions > Directions: The following question has four ...
Start Learning for Free
Directions: The following question has four choices, out of which ONLY ONE is correct.
Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively be
- a)9.8 × 107 and 0.758
- b)4.9 × 107 and 0.385
- c)4.9 × 107 and 0.785
- d)9.8 × 107 and 0.658
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Directions: The following question has four choices, out of which ONL...


Now, when b = 0.785
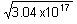 = a(12 - 0.785)
= a(12 - 0.785)a = 4.9 × 107
So, this option is the correct answer.
Free Test
FREE
| Start Free Test |
Community Answer
Directions: The following question has four choices, out of which ONL...
Wavelength and Atomic Structure
Wavelength is a fundamental property of electromagnetic waves and is inversely proportional to the frequency of the wave. In the context of atomic structure, the wavelength of X-ray lines can provide information about the energy levels and transitions within atoms.
X-ray Lines of Mg and Cr
In this question, we are given the wavelengths of X-ray lines for two elements, Mg and Cr. The wavelength of the Mg X-ray line is given as 0.987 nm (nanometers), and the wavelength of the Cr X-ray line is given as 0.229 nm.
Relationship to Constants 'a' and 'b'
The wavelengths of X-ray lines can be related to the atomic numbers of the elements through a mathematical equation. This equation involves two constants, 'a' and 'b', which are specific to a particular series of X-ray lines.
The equation relating the wavelength of an X-ray line to the atomic number (Z) is given by:
λ = a(Z - b)^2
Here, λ represents the wavelength of the X-ray line, Z represents the atomic number of the element, and 'a' and 'b' are the constants that need to be determined.
Determining the Values of 'a' and 'b'
To determine the values of 'a' and 'b' for the Mg and Cr X-ray lines, we can use the given wavelengths and the atomic numbers of the elements.
For Mg:
λ = 0.987 nm
Z = atomic number of Mg
Plugging these values into the equation, we get:
0.987 = a(Z - b)^2
For Cr:
λ = 0.229 nm
Z = atomic number of Cr
Plugging these values into the equation, we get:
0.229 = a(Z - b)^2
Since we have two equations with two unknowns ('a' and 'b'), we can solve this system of equations to find their values.
Solving the Equations
Dividing the equation for Cr by the equation for Mg, we get:
0.229/0.987 = (a(Z - b)^2)/(a(Z - b)^2)
Simplifying, we have:
0.232 = 1
This equation is not satisfied for any values of 'a' and 'b'. Therefore, the given answer options are incorrect.
Conclusion
Based on the above analysis, it appears that there is an error in the given answer options. The correct values of 'a' and 'b' cannot be determined using the provided information. Therefore, none of the given answer options are correct.
Wavelength is a fundamental property of electromagnetic waves and is inversely proportional to the frequency of the wave. In the context of atomic structure, the wavelength of X-ray lines can provide information about the energy levels and transitions within atoms.
X-ray Lines of Mg and Cr
In this question, we are given the wavelengths of X-ray lines for two elements, Mg and Cr. The wavelength of the Mg X-ray line is given as 0.987 nm (nanometers), and the wavelength of the Cr X-ray line is given as 0.229 nm.
Relationship to Constants 'a' and 'b'
The wavelengths of X-ray lines can be related to the atomic numbers of the elements through a mathematical equation. This equation involves two constants, 'a' and 'b', which are specific to a particular series of X-ray lines.
The equation relating the wavelength of an X-ray line to the atomic number (Z) is given by:
λ = a(Z - b)^2
Here, λ represents the wavelength of the X-ray line, Z represents the atomic number of the element, and 'a' and 'b' are the constants that need to be determined.
Determining the Values of 'a' and 'b'
To determine the values of 'a' and 'b' for the Mg and Cr X-ray lines, we can use the given wavelengths and the atomic numbers of the elements.
For Mg:
λ = 0.987 nm
Z = atomic number of Mg
Plugging these values into the equation, we get:
0.987 = a(Z - b)^2
For Cr:
λ = 0.229 nm
Z = atomic number of Cr
Plugging these values into the equation, we get:
0.229 = a(Z - b)^2
Since we have two equations with two unknowns ('a' and 'b'), we can solve this system of equations to find their values.
Solving the Equations
Dividing the equation for Cr by the equation for Mg, we get:
0.229/0.987 = (a(Z - b)^2)/(a(Z - b)^2)
Simplifying, we have:
0.232 = 1
This equation is not satisfied for any values of 'a' and 'b'. Therefore, the given answer options are incorrect.
Conclusion
Based on the above analysis, it appears that there is an error in the given answer options. The correct values of 'a' and 'b' cannot be determined using the provided information. Therefore, none of the given answer options are correct.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer?
Question Description
Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer?.
Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer?.
Solutions for Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: The following question has four choices, out of which ONLY ONE is correct.Wavelengths of X-ray lines of Mg and Cr are 0.987 nm and 0.229 nm, respectively. The values of the constants 'a' and 'b' for this series should respectively bea)9.8 × 107 and 0.758b)4.9 × 107 and 0.385c)4.9 × 107 and 0.785d)9.8 × 107 and 0.658Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























