IIT JAM Exam > IIT JAM Questions > What is meant by the abnormal transport numbe...
Start Learning for Free
What is meant by the abnormal transport number of an ion?
Verified Answer
What is meant by the abnormal transport number of an ion?
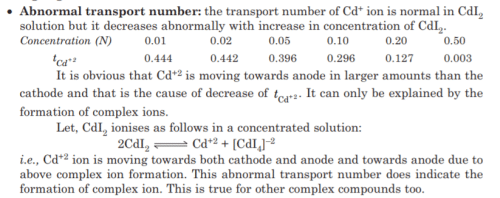
 This question is part of UPSC exam. View all IIT JAM courses
This question is part of UPSC exam. View all IIT JAM courses
Most Upvoted Answer
What is meant by the abnormal transport number of an ion?
Abnormal Transport Number of an Ion:
The transport number of an ion represents the fraction of the total current carried by that ion in an electrolyte solution. It is a measure of the relative mobility of an ion compared to other ions in the solution. The transport number is influenced by factors such as the size, charge, and concentration of the ions, as well as the nature of the solvent.
Factors Affecting Transport Number:
Several factors contribute to the abnormal transport number of an ion:
1. Size and Charge: The size and charge of an ion determine its ability to move through a solution. Smaller ions and ions with higher charge tend to have higher transport numbers because they experience less resistance from the surrounding solvent molecules.
2. Ion Concentration: The concentration of ions in a solution affects their transport numbers. At low concentrations, ions have higher transport numbers as they are less likely to collide with other ions. However, at high concentrations, the transport numbers may decrease due to increased ion-ion interactions.
3. Solvent Properties: The nature of the solvent also influences the transport numbers. Different solvents have different dielectric constants, viscosity, and other properties that can affect the movement of ions. For example, a solvent with a high dielectric constant can weaken the ion-solvent interactions, allowing ions to move more freely and exhibit higher transport numbers.
4. Ion-Solvent Interactions: The strength of the interactions between ions and the solvent affects their transport numbers. If the interactions are weak, ions can move more easily and exhibit higher transport numbers. Conversely, strong ion-solvent interactions can hinder ion movement and result in lower transport numbers.
Significance of Abnormal Transport Number:
The abnormal transport number of an ion is of particular interest in electrochemical processes and battery technologies. It plays a crucial role in determining the efficiency of charge transfer and the overall performance of electrochemical systems. Understanding the abnormal transport number helps in designing and optimizing battery systems, fuel cells, and other electrochemical devices.
Applications:
1. Battery Technology: The transport number of ions in battery electrolytes influences the charging and discharging rates, as well as the overall energy storage capacity of the battery.
2. Fuel Cells: Transport numbers affect the efficiency of ion transport and the production of electrical energy in fuel cells.
3. Electroplating: The transport number of metal ions in electrolyte solutions determines the deposition rate and quality of electroplated coatings.
4. Electrolysis: The transport numbers of ions in the electrolyte solution impact the efficiency and selectivity of chemical reactions during electrolysis processes.
In conclusion, the transport number of an ion represents its relative mobility in an electrolyte solution. It is influenced by factors such as ion size, charge, concentration, solvent properties, and ion-solvent interactions. The abnormal transport number has significant implications in various electrochemical processes, battery technologies, and other applications involving ion transport.
The transport number of an ion represents the fraction of the total current carried by that ion in an electrolyte solution. It is a measure of the relative mobility of an ion compared to other ions in the solution. The transport number is influenced by factors such as the size, charge, and concentration of the ions, as well as the nature of the solvent.
Factors Affecting Transport Number:
Several factors contribute to the abnormal transport number of an ion:
1. Size and Charge: The size and charge of an ion determine its ability to move through a solution. Smaller ions and ions with higher charge tend to have higher transport numbers because they experience less resistance from the surrounding solvent molecules.
2. Ion Concentration: The concentration of ions in a solution affects their transport numbers. At low concentrations, ions have higher transport numbers as they are less likely to collide with other ions. However, at high concentrations, the transport numbers may decrease due to increased ion-ion interactions.
3. Solvent Properties: The nature of the solvent also influences the transport numbers. Different solvents have different dielectric constants, viscosity, and other properties that can affect the movement of ions. For example, a solvent with a high dielectric constant can weaken the ion-solvent interactions, allowing ions to move more freely and exhibit higher transport numbers.
4. Ion-Solvent Interactions: The strength of the interactions between ions and the solvent affects their transport numbers. If the interactions are weak, ions can move more easily and exhibit higher transport numbers. Conversely, strong ion-solvent interactions can hinder ion movement and result in lower transport numbers.
Significance of Abnormal Transport Number:
The abnormal transport number of an ion is of particular interest in electrochemical processes and battery technologies. It plays a crucial role in determining the efficiency of charge transfer and the overall performance of electrochemical systems. Understanding the abnormal transport number helps in designing and optimizing battery systems, fuel cells, and other electrochemical devices.
Applications:
1. Battery Technology: The transport number of ions in battery electrolytes influences the charging and discharging rates, as well as the overall energy storage capacity of the battery.
2. Fuel Cells: Transport numbers affect the efficiency of ion transport and the production of electrical energy in fuel cells.
3. Electroplating: The transport number of metal ions in electrolyte solutions determines the deposition rate and quality of electroplated coatings.
4. Electrolysis: The transport numbers of ions in the electrolyte solution impact the efficiency and selectivity of chemical reactions during electrolysis processes.
In conclusion, the transport number of an ion represents its relative mobility in an electrolyte solution. It is influenced by factors such as ion size, charge, concentration, solvent properties, and ion-solvent interactions. The abnormal transport number has significant implications in various electrochemical processes, battery technologies, and other applications involving ion transport.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
What is meant by the abnormal transport number of an ion?
Question Description
What is meant by the abnormal transport number of an ion? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about What is meant by the abnormal transport number of an ion? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is meant by the abnormal transport number of an ion?.
What is meant by the abnormal transport number of an ion? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about What is meant by the abnormal transport number of an ion? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is meant by the abnormal transport number of an ion?.
Solutions for What is meant by the abnormal transport number of an ion? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of What is meant by the abnormal transport number of an ion? defined & explained in the simplest way possible. Besides giving the explanation of
What is meant by the abnormal transport number of an ion?, a detailed solution for What is meant by the abnormal transport number of an ion? has been provided alongside types of What is meant by the abnormal transport number of an ion? theory, EduRev gives you an
ample number of questions to practice What is meant by the abnormal transport number of an ion? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















