UGC NET Exam > UGC NET Questions > Of the following, the correct statements abou...
Start Learning for Free
Of the following, the correct statements about carboxypeptidase-A are
A. Zn2+ ion acts as a Lewis acid.
B. The substitution of Zn2+ ion by Co2+ ion renders the enzyme inactive.
C. Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ ion.
D. Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ ion.
A. Zn2+ ion acts as a Lewis acid.
B. The substitution of Zn2+ ion by Co2+ ion renders the enzyme inactive.
C. Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ ion.
D. Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ ion.
- a)A and C only
- b)A, C, and D only
- c)B and D only
- d)A and B only
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Of the following, the correct statements about carboxypeptidase-A areA...
- Carboxypeptidase-A is a metalloenzyme that requires a zinc ion (Zn2+) as a cofactor for its catalytic activity. The coordination of the zinc ion by specific amino acid residues is essential for the enzyme's function.
- The active site of Carboxypeptidase-A is shown below:
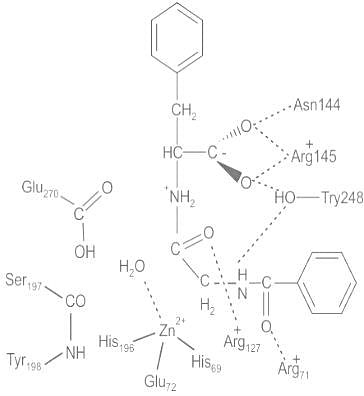
- Carboxypeptidase A (CPA) contains a zinc (Zn2+) metal center in a tetrahedral geometry with amino acid residues in close proximity around zinc to facilitate catalysis and binding.
- Out of the 307 amino acids bonded in a peptide chain, the following amino acid residues are important for catalysis and binding; Glu-270, Arg-71, Arg-127, Asn-144, Arg-145, and Tyr-248.
- The above figure illustrates the tetrahedral zinc complex active site with the important amino acid residues that surround the complex.
- The zinc metal is a strong electrophilic Lewis acid catalyst which stabilizes a coordinated water molecule as well as stabilizes the negative intermediates that occur throughout the hydrolytic reaction.
- Stabilization of both the coordinated water molecule and negative intermediates are assisted by polar residues in the active site which are in close proximity to facilitate hydrogen bonding.
- The active site can be characterized into two sub-sites denoted as S1’ and S1. The S1’ sub-site is the hydrophobic pocket of the enzyme, and Tyr-248 acts to ‘cap’ the hydrophobic pocket after substrate or inhibitor is bound (SITE).
- The hydrogen bonding from the hydroxyl group in Tyr-248 facilitates this conformation due to interaction with the terminal carboxylates of substrates that bind. Substantial movement is required for this enzyme and induced fit model explains how this interaction occurs.
Carboxypeptidase A (CPA) contains a zinc (Zn2+) metal center in a tetrahedral geometry with amino acid residues in close proximity around zinc to facilitate catalysis and binding.
The zinc metal is a strong electrophilic Lewis acid catalyst which stabilizes a coordinated water molecule as well as stabilizes the negative intermediates that occur throughout the hydrolytic reaction.
Thus, statement A is correct.
Option B, which suggests that the substitution of the Zn2+ ion by Co2+ ion renders the enzyme inactive, is incorrect. While the substitution of Zn2+ by Co2+ may impact the enzyme's activity, it does not necessarily render it completely inactive. The activity of the enzyme may be altered, but it can still retain some functionality.
Statment C is correct as two histidine nitrogen atoms, glutamate oxygen atom(s), and a water molecule coordinate to a Zn2+ ion in carboxypeptidase-A.
Option D, which states that three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ ion, is incorrect. Carboxypeptidase-A typically coordinates with two histidine nitrogen atoms, a glutamate oxygen atom(s), and a water molecule to the Zn2+ ion. The two histidines and the glutamate residue provide important ligands for binding and activating the zinc ion.
Hence, the correct statements about carboxypeptidase-A are A and C only.
The zinc metal is a strong electrophilic Lewis acid catalyst which stabilizes a coordinated water molecule as well as stabilizes the negative intermediates that occur throughout the hydrolytic reaction.
Thus, statement A is correct.
Option B, which suggests that the substitution of the Zn2+ ion by Co2+ ion renders the enzyme inactive, is incorrect. While the substitution of Zn2+ by Co2+ may impact the enzyme's activity, it does not necessarily render it completely inactive. The activity of the enzyme may be altered, but it can still retain some functionality.
Statment C is correct as two histidine nitrogen atoms, glutamate oxygen atom(s), and a water molecule coordinate to a Zn2+ ion in carboxypeptidase-A.
Option D, which states that three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ ion, is incorrect. Carboxypeptidase-A typically coordinates with two histidine nitrogen atoms, a glutamate oxygen atom(s), and a water molecule to the Zn2+ ion. The two histidines and the glutamate residue provide important ligands for binding and activating the zinc ion.
Hence, the correct statements about carboxypeptidase-A are A and C only.
Free Test
FREE
| Start Free Test |
Community Answer
Of the following, the correct statements about carboxypeptidase-A areA...
Statement A: Zn2 ion acts as a Lewis acid.
Statement B: The substitution of Zn2 ion by Co2 ion renders the enzyme inactive.
Statement C: Two histidine nitrogen atoms, glutamate oxygen atom(s), and a water molecule coordinate to a Zn2 ion.
Statement D: Three histidine nitrogen atoms and a water molecule coordinate to a Zn2 ion.
Explanation:
Carboxypeptidase-A:
Carboxypeptidase-A is a metalloenzyme that catalyzes the hydrolysis of the peptide bond at the C-terminal end of a protein or peptide. It is classified as an exopeptidase because it cleaves peptide bonds one at a time from the C-terminal end of a polypeptide chain.
Statement A: Zn2 ion acts as a Lewis acid.
Explanation:
Zn2 ion in carboxypeptidase-A acts as a Lewis acid, which means it can accept a pair of electrons from a Lewis base. In the active site of the enzyme, the Zn2 ion coordinates with several amino acid residues, including histidine and glutamate, to form a catalytic center. The Zn2 ion acts as an electrophile, stabilizing the negative charge that develops during the hydrolysis reaction.
Statement B: The substitution of Zn2 ion by Co2 ion renders the enzyme inactive.
Explanation:
The substitution of Zn2 ion by Co2 ion in carboxypeptidase-A can render the enzyme inactive. This is because the specific coordination and electronic properties of Zn2 ion are necessary for the enzyme to function properly. Co2 ion may not have the same coordination geometry or catalytic properties as Zn2 ion, leading to a loss of enzymatic activity.
Statement C: Two histidine nitrogen atoms, glutamate oxygen atom(s), and a water molecule coordinate to a Zn2 ion.
Explanation:
In the active site of carboxypeptidase-A, two histidine nitrogen atoms, a glutamate oxygen atom(s), and a water molecule coordinate to a Zn2 ion. These amino acid residues form a coordination complex with the Zn2 ion, providing the necessary environment for catalysis. The histidine residues act as ligands, donating electron pairs to the Zn2 ion, while the glutamate residue and water molecule provide additional coordination and stabilization.
Statement D: Three histidine nitrogen atoms and a water molecule coordinate to a Zn2 ion.
Explanation:
This statement is incorrect. Carboxypeptidase-A does not coordinate with three histidine nitrogen atoms and a water molecule to a Zn2 ion. It is the combination of two histidine nitrogen atoms, a glutamate oxygen atom(s), and a water molecule that coordinate to the Zn2 ion in the active site of the enzyme.
Conclusion:
From the given statements, the correct statements about carboxypeptidase-A are A. Zn2 ion acts as a Lewis acid. Therefore, the correct answer is option 'A'.
Statement B: The substitution of Zn2 ion by Co2 ion renders the enzyme inactive.
Statement C: Two histidine nitrogen atoms, glutamate oxygen atom(s), and a water molecule coordinate to a Zn2 ion.
Statement D: Three histidine nitrogen atoms and a water molecule coordinate to a Zn2 ion.
Explanation:
Carboxypeptidase-A:
Carboxypeptidase-A is a metalloenzyme that catalyzes the hydrolysis of the peptide bond at the C-terminal end of a protein or peptide. It is classified as an exopeptidase because it cleaves peptide bonds one at a time from the C-terminal end of a polypeptide chain.
Statement A: Zn2 ion acts as a Lewis acid.
Explanation:
Zn2 ion in carboxypeptidase-A acts as a Lewis acid, which means it can accept a pair of electrons from a Lewis base. In the active site of the enzyme, the Zn2 ion coordinates with several amino acid residues, including histidine and glutamate, to form a catalytic center. The Zn2 ion acts as an electrophile, stabilizing the negative charge that develops during the hydrolysis reaction.
Statement B: The substitution of Zn2 ion by Co2 ion renders the enzyme inactive.
Explanation:
The substitution of Zn2 ion by Co2 ion in carboxypeptidase-A can render the enzyme inactive. This is because the specific coordination and electronic properties of Zn2 ion are necessary for the enzyme to function properly. Co2 ion may not have the same coordination geometry or catalytic properties as Zn2 ion, leading to a loss of enzymatic activity.
Statement C: Two histidine nitrogen atoms, glutamate oxygen atom(s), and a water molecule coordinate to a Zn2 ion.
Explanation:
In the active site of carboxypeptidase-A, two histidine nitrogen atoms, a glutamate oxygen atom(s), and a water molecule coordinate to a Zn2 ion. These amino acid residues form a coordination complex with the Zn2 ion, providing the necessary environment for catalysis. The histidine residues act as ligands, donating electron pairs to the Zn2 ion, while the glutamate residue and water molecule provide additional coordination and stabilization.
Statement D: Three histidine nitrogen atoms and a water molecule coordinate to a Zn2 ion.
Explanation:
This statement is incorrect. Carboxypeptidase-A does not coordinate with three histidine nitrogen atoms and a water molecule to a Zn2 ion. It is the combination of two histidine nitrogen atoms, a glutamate oxygen atom(s), and a water molecule that coordinate to the Zn2 ion in the active site of the enzyme.
Conclusion:
From the given statements, the correct statements about carboxypeptidase-A are A. Zn2 ion acts as a Lewis acid. Therefore, the correct answer is option 'A'.
Attention UGC NET Students!
To make sure you are not studying endlessly, EduRev has designed UGC NET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in UGC NET.

|
Explore Courses for UGC NET exam
|

|
Similar UGC NET Doubts
Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer?
Question Description
Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? for UGC NET 2024 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for UGC NET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer?.
Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? for UGC NET 2024 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for UGC NET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Of the following, the correct statements about carboxypeptidase-A areA. Zn2+ion acts as a Lewis acid.B. The substitution of Zn2+ion by Co2+ion renders the enzyme inactive.C.Two histidine nitrogen atoms, glutamate oxygen atom(s) and a water molecule coordinate to a Zn2+ion.D.Three histidine nitrogen atoms and a water molecule coordinate to a Zn2+ion.a)A and C onlyb)A, C, and D onlyc)B and D onlyd)A and B onlyCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























