UGC NET Exam > UGC NET Questions > The possible terms arising from a p1d1configu...
Start Learning for Free
The possible terms arising from a p1d1 configuration are
- a)1F and 2D
- b)3F and 3D
- c)3F and 1D
- d)3F and 1F
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
The possible terms arising from a p1d1configuration area)1F and2Db)3F ...
Term symbols usually represent electronic states in the Russell-Saunders coupling scheme, where a typical atomic term symbol consists of the spin multiplicity, the symmetry label and the total angular momentum of the atom. They have the format of 2S+1LJ
The method of using a table to count possible "microstates" has been developed so long ago and honed by so many scientists and educators that it is hard to accredit a single person.
Let's take the electronic configuration of d3 as an example. In the Slater's table, each cell contains the number of ways to assign the three electrons quantum numbers according to the Ms and ML values.
These assignments follow Pauli's exclusion law. The figure below shows an example to find out how many ways to assign quantum numbers to d3 electrons when ML = 3 and Ms = -1/2.
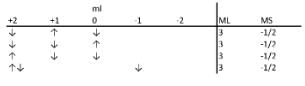
The correct answer is option 3.
The method of using a table to count possible "microstates" has been developed so long ago and honed by so many scientists and educators that it is hard to accredit a single person.
Let's take the electronic configuration of d3 as an example. In the Slater's table, each cell contains the number of ways to assign the three electrons quantum numbers according to the Ms and ML values.
These assignments follow Pauli's exclusion law. The figure below shows an example to find out how many ways to assign quantum numbers to d3 electrons when ML = 3 and Ms = -1/2.


The correct answer is option 3.
Most Upvoted Answer
The possible terms arising from a p1d1configuration area)1F and2Db)3F ...
Understanding p1d1 Configuration
The p1d1 configuration refers to the arrangement of electrons in the atomic orbitals. The specific arrangement in question allows us to derive the possible terms.
Terms Derived from p1d1 Configuration
To analyze the terms, we consider the following:
- p Orbital (1 electron): The p orbital can have a maximum of 3 orbitals (px, py, pz) and holds one electron in this case. The possible angular momentum (L) states are:
- L = 1 (p state)
- d Orbital (1 electron): The d orbital can have a maximum of 5 orbitals (dxy, dyz, dzx, dx2-y2, dz2) and also holds one electron here. The possible angular momentum states are:
- L = 2 (d state)
Combining the Angular Momentum States
When combining the p and d states, we use the vector coupling of angular momentum:
- The total angular momentum (L) can be calculated as:
- L can range from |L1 - L2| to |L1 + L2|, where L1 is from the p orbital (1) and L2 is from the d orbital (2).
Thus, the possible values for the total angular momentum (L) are:
- L = 1 (p) + L = 2 (d):
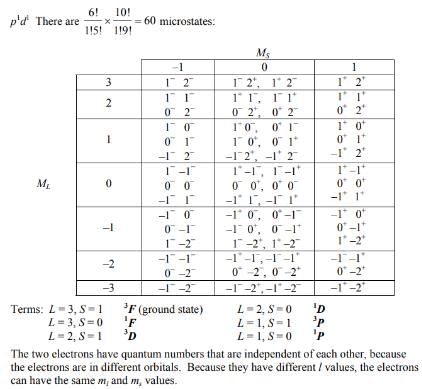
- Possible values: L = 1, 2, 3 (which corresponds to terms: P, D, F)
Possible Terms and Their Multiplicities
- 3F: This term arises from the maximum multiplicity due to two unpaired electrons.
- 1D: This term arises when the total spin is paired.
- 1F: This is a singlet state with a total spin of S = 0.
Given these combinations, the possible terms from the p1d1 configuration can be summarized as:
- 3F
- 1D
Conclusion
Therefore, the correct answer from the options provided is 3F and 1D, which corresponds to option 'C'.
The p1d1 configuration refers to the arrangement of electrons in the atomic orbitals. The specific arrangement in question allows us to derive the possible terms.
Terms Derived from p1d1 Configuration
To analyze the terms, we consider the following:
- p Orbital (1 electron): The p orbital can have a maximum of 3 orbitals (px, py, pz) and holds one electron in this case. The possible angular momentum (L) states are:
- L = 1 (p state)
- d Orbital (1 electron): The d orbital can have a maximum of 5 orbitals (dxy, dyz, dzx, dx2-y2, dz2) and also holds one electron here. The possible angular momentum states are:
- L = 2 (d state)
Combining the Angular Momentum States
When combining the p and d states, we use the vector coupling of angular momentum:
- The total angular momentum (L) can be calculated as:
- L can range from |L1 - L2| to |L1 + L2|, where L1 is from the p orbital (1) and L2 is from the d orbital (2).
Thus, the possible values for the total angular momentum (L) are:
- L = 1 (p) + L = 2 (d):
- Possible values: L = 1, 2, 3 (which corresponds to terms: P, D, F)
Possible Terms and Their Multiplicities
- 3F: This term arises from the maximum multiplicity due to two unpaired electrons.
- 1D: This term arises when the total spin is paired.
- 1F: This is a singlet state with a total spin of S = 0.
Given these combinations, the possible terms from the p1d1 configuration can be summarized as:
- 3F
- 1D
Conclusion
Therefore, the correct answer from the options provided is 3F and 1D, which corresponds to option 'C'.

|
Explore Courses for UGC NET exam
|

|
The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer?
Question Description
The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer?.
The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? for UGC NET 2025 is part of UGC NET preparation. The Question and answers have been prepared according to the UGC NET exam syllabus. Information about The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UGC NET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for UGC NET.
Download more important topics, notes, lectures and mock test series for UGC NET Exam by signing up for free.
Here you can find the meaning of The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The possible terms arising from a p1d1configuration area)1F and2Db)3F and3Dc)3F and1Dd)3F and1FCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice UGC NET tests.

|
Explore Courses for UGC NET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.






















