JEE Exam > JEE Questions > The coupling of diazonium salt of 4-amino ben...
Start Learning for Free
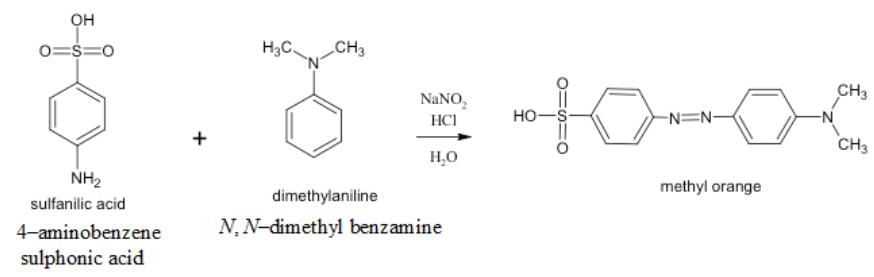
The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine produces
- a)Phenolphthalein
- b)Methyl red
- c)Methyl orange
- d)Litmus
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
The coupling of diazonium salt of 4-amino benzene sulphonic acid with ...
The coupling of diazonium salt of 4-aminobenzene sulphonic acid with N, N-dimethyl benzamine produces methyl orange.

Most Upvoted Answer
The coupling of diazonium salt of 4-amino benzene sulphonic acid with ...
The coupling reaction between the diazonium salt of 4-amino benzene sulphonic acid and N, N-dimethyl benzamine produces Methyl orange.
Explanation:
- The diazonium salt of 4-amino benzene sulphonic acid is formed by the reaction between 4-amino benzene sulphonic acid and sodium nitrite in the presence of hydrochloric acid. This reaction is known as diazotization.
- The diazonium salt is highly reactive and can undergo coupling reactions with various compounds. In this case, it reacts with N, N-dimethyl benzamine to form Methyl orange.
- Methyl orange is an azo dye and is commonly used as an acid-base indicator. It is orange in acidic solutions and turns red in basic solutions.
- The reaction between the diazonium salt and N, N-dimethyl benzamine involves the formation of an azo compound. The diazonium group (-N2+) is replaced by the N, N-dimethyl benzamine moiety, resulting in the formation of Methyl orange.
- The reaction can be represented as follows:
diazonium salt of 4-amino benzene sulphonic acid + N, N-dimethyl benzamine → Methyl orange
- Methyl orange is widely used in analytical chemistry and is one of the most commonly used acid-base indicators. It is used to determine the pH of a solution by observing the color change.
- In acidic solutions, Methyl orange is protonated and exists in its orange form. In basic solutions, it loses a proton and exists in its red form.
- The color change of Methyl orange is due to the different electronic states of the azo group in acidic and basic environments. In an acidic solution, the azo group is in the more stable hydrazone form, resulting in an orange color. In a basic solution, the azo group is in the less stable azo form, resulting in a red color.
- Therefore, the correct answer is option C, Methyl orange.
Explanation:
- The diazonium salt of 4-amino benzene sulphonic acid is formed by the reaction between 4-amino benzene sulphonic acid and sodium nitrite in the presence of hydrochloric acid. This reaction is known as diazotization.
- The diazonium salt is highly reactive and can undergo coupling reactions with various compounds. In this case, it reacts with N, N-dimethyl benzamine to form Methyl orange.
- Methyl orange is an azo dye and is commonly used as an acid-base indicator. It is orange in acidic solutions and turns red in basic solutions.
- The reaction between the diazonium salt and N, N-dimethyl benzamine involves the formation of an azo compound. The diazonium group (-N2+) is replaced by the N, N-dimethyl benzamine moiety, resulting in the formation of Methyl orange.
- The reaction can be represented as follows:
diazonium salt of 4-amino benzene sulphonic acid + N, N-dimethyl benzamine → Methyl orange
- Methyl orange is widely used in analytical chemistry and is one of the most commonly used acid-base indicators. It is used to determine the pH of a solution by observing the color change.
- In acidic solutions, Methyl orange is protonated and exists in its orange form. In basic solutions, it loses a proton and exists in its red form.
- The color change of Methyl orange is due to the different electronic states of the azo group in acidic and basic environments. In an acidic solution, the azo group is in the more stable hydrazone form, resulting in an orange color. In a basic solution, the azo group is in the less stable azo form, resulting in a red color.
- Therefore, the correct answer is option C, Methyl orange.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer?
Question Description
The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer?.
The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The coupling of diazonium salt of 4-amino benzene sulphonic acid with N, N-dimethyl benzamine producesa)Phenolphthaleinb)Methyl redc)Methyl oranged)LitmusCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























