Class 12 Exam > Class 12 Questions > Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)...
Start Learning for Free
Type of bonding in K4 [Fe(CN)6] is/a
- a)ionic
- b)covalent
- c)metallic
- d)coordinate covalent
Correct answer is option 'A,B,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coord...
The complex K4[Fe(CN)6] whose formula can be written like that of double salt. Fe(CN)2 . 4KCN, dissociates to give K+ and [Fe(CN)6]4- ions in the aqueous solution.
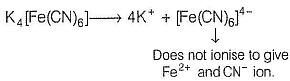
Most Upvoted Answer
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coord...
Ionic Bonding:
- In K4[Fe(CN)6], the potassium ions (K+) and the cyanide ions (CN-) are involved in ionic bonding.
- Ionic bonding occurs between a metal and a non-metal, where one atom donates electrons to another atom to form a bond.
- In this compound, the potassium ions donate electrons to the cyanide ions, resulting in the formation of ionic bonds between them.
- The potassium ions lose one electron each and become positively charged, while the cyanide ions gain one electron each and become negatively charged.
- The opposite charges of the ions attract each other and hold the compound together.
Covalent Bonding:
- In K4[Fe(CN)6], the iron atom (Fe) and the carbon and nitrogen atoms in the cyanide group (CN) are involved in covalent bonding.
- Covalent bonding occurs between non-metal atoms, where the atoms share electrons to form a bond.
- In this compound, the iron atom forms covalent bonds with the carbon and nitrogen atoms in the cyanide group.
- The carbon and nitrogen atoms share their electrons with the iron atom, resulting in the formation of covalent bonds.
- Covalent bonding allows for the sharing of electrons between atoms, creating a stable structure.
Coordinate Covalent Bonding:
- In K4[Fe(CN)6], the iron atom (Fe) and the cyanide ions (CN-) are involved in coordinate covalent bonding.
- Coordinate covalent bonding occurs when one atom donates a pair of electrons to another atom to form a bond.
- In this compound, the cyanide ions donate a pair of electrons to the iron atom, resulting in the formation of coordinate covalent bonds.
- The cyanide ions act as a Lewis base by donating their electron pair to the iron atom, which acts as a Lewis acid by accepting the electron pair.
- Coordinate covalent bonding allows for the formation of complex compounds with a central metal ion bonded to various ligands.
In summary, K4[Fe(CN)6] exhibits ionic bonding between the potassium ions and the cyanide ions, covalent bonding between the iron atom and the carbon and nitrogen atoms in the cyanide group, and coordinate covalent bonding between the iron atom and the cyanide ions.
- In K4[Fe(CN)6], the potassium ions (K+) and the cyanide ions (CN-) are involved in ionic bonding.
- Ionic bonding occurs between a metal and a non-metal, where one atom donates electrons to another atom to form a bond.
- In this compound, the potassium ions donate electrons to the cyanide ions, resulting in the formation of ionic bonds between them.
- The potassium ions lose one electron each and become positively charged, while the cyanide ions gain one electron each and become negatively charged.
- The opposite charges of the ions attract each other and hold the compound together.
Covalent Bonding:
- In K4[Fe(CN)6], the iron atom (Fe) and the carbon and nitrogen atoms in the cyanide group (CN) are involved in covalent bonding.
- Covalent bonding occurs between non-metal atoms, where the atoms share electrons to form a bond.
- In this compound, the iron atom forms covalent bonds with the carbon and nitrogen atoms in the cyanide group.
- The carbon and nitrogen atoms share their electrons with the iron atom, resulting in the formation of covalent bonds.
- Covalent bonding allows for the sharing of electrons between atoms, creating a stable structure.
Coordinate Covalent Bonding:
- In K4[Fe(CN)6], the iron atom (Fe) and the cyanide ions (CN-) are involved in coordinate covalent bonding.
- Coordinate covalent bonding occurs when one atom donates a pair of electrons to another atom to form a bond.
- In this compound, the cyanide ions donate a pair of electrons to the iron atom, resulting in the formation of coordinate covalent bonds.
- The cyanide ions act as a Lewis base by donating their electron pair to the iron atom, which acts as a Lewis acid by accepting the electron pair.
- Coordinate covalent bonding allows for the formation of complex compounds with a central metal ion bonded to various ligands.
In summary, K4[Fe(CN)6] exhibits ionic bonding between the potassium ions and the cyanide ions, covalent bonding between the iron atom and the carbon and nitrogen atoms in the cyanide group, and coordinate covalent bonding between the iron atom and the cyanide ions.
Free Test
FREE
| Start Free Test |
Community Answer
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coord...
Plz some one explain this question because i think option 'c' is also correct becoz in this question fe is bonded with the ligand CN

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer?
Question Description
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer?.
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer?.
Solutions for Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer?, a detailed solution for Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? has been provided alongside types of Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Type of bonding in K4 [Fe(CN)6] is/aa)ionicb)covalentc)metallicd)coordinate covalentCorrect answer is option 'A,B,D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















