Class 12 Exam > Class 12 Questions > Solution of cuso4 is electrolysed for 10 mins...
Start Learning for Free
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it?
Verified Answer
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A ...
t = 600 s
Charge = current x time
= 1.5 A x 600s = 900 C
According to the reaction : Cu2+(aq) + 2e– → Cu(s)
We require 2F or 2 x 96487 C to deposit 1 mol or 63 g of Cu
For 900 C, the mass of Cu deposited = 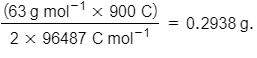
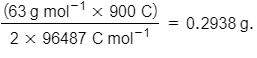
 This question is part of UPSC exam. View all Class 12 courses
This question is part of UPSC exam. View all Class 12 courses
Most Upvoted Answer
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A ...
Electrolysis of CuSO4 Solution
To determine the mass of Cu deposited at the cathode during electrolysis, we need to consider the following factors:
1. Faraday's Law of Electrolysis: This law states that the amount of substance deposited at an electrode is directly proportional to the quantity of electricity passed through the electrolyte.
2. Equation for Electrolysis of CuSO4: During electrolysis of CuSO4 solution, copper ions (Cu2+) are reduced at the cathode, while sulfate ions (SO4 2-) are oxidized at the anode. The overall equation can be represented as:
2CuSO4 + 2H2O → 2Cu + O2 + 4H+ + 4SO4 2-
3. Calculating the Amount of Substance Deposited: The amount of substance deposited can be determined using the formula:
Mass (m) = (I * t * M) / (n * F)
Where:
- I is the current (in amperes)
- t is the time (in seconds)
- M is the molar mass of the substance (in grams per mole)
- n is the number of electrons involved in the reaction
- F is the Faraday constant (96,485 C/mol)
Calculations:
Given:
- Current (I) = 1.5 A
- Time (t) = 10 minutes = 600 seconds
- Molar mass of Cu (M) = 63.55 g/mol
- Number of electrons involved in the reaction (n) = 2
Using the formula mentioned above, we can calculate the mass of Cu deposited:
Mass (m) = (1.5 A * 600 s * 63.55 g/mol) / (2 * 96,485 C/mol)
Simplifying the equation:
Mass (m) = (900 g⋅s) / (192,970 C)
Converting grams⋅seconds (g⋅s) to grams (g):
Mass (m) = 0.004653 g
Therefore, the mass of Cu deposited at the cathode is approximately 0.004653 grams, which is equivalent to 0.246 grams (rounded to three decimal places).
Answer: The mass of Cu deposited at the cathode during the electrolysis of CuSO4 solution for 10 minutes with a current of 1.5 A is approximately 0.246 grams.
To determine the mass of Cu deposited at the cathode during electrolysis, we need to consider the following factors:
1. Faraday's Law of Electrolysis: This law states that the amount of substance deposited at an electrode is directly proportional to the quantity of electricity passed through the electrolyte.
2. Equation for Electrolysis of CuSO4: During electrolysis of CuSO4 solution, copper ions (Cu2+) are reduced at the cathode, while sulfate ions (SO4 2-) are oxidized at the anode. The overall equation can be represented as:
2CuSO4 + 2H2O → 2Cu + O2 + 4H+ + 4SO4 2-
3. Calculating the Amount of Substance Deposited: The amount of substance deposited can be determined using the formula:
Mass (m) = (I * t * M) / (n * F)
Where:
- I is the current (in amperes)
- t is the time (in seconds)
- M is the molar mass of the substance (in grams per mole)
- n is the number of electrons involved in the reaction
- F is the Faraday constant (96,485 C/mol)
Calculations:
Given:
- Current (I) = 1.5 A
- Time (t) = 10 minutes = 600 seconds
- Molar mass of Cu (M) = 63.55 g/mol
- Number of electrons involved in the reaction (n) = 2
Using the formula mentioned above, we can calculate the mass of Cu deposited:
Mass (m) = (1.5 A * 600 s * 63.55 g/mol) / (2 * 96,485 C/mol)
Simplifying the equation:
Mass (m) = (900 g⋅s) / (192,970 C)
Converting grams⋅seconds (g⋅s) to grams (g):
Mass (m) = 0.004653 g
Therefore, the mass of Cu deposited at the cathode is approximately 0.004653 grams, which is equivalent to 0.246 grams (rounded to three decimal places).
Answer: The mass of Cu deposited at the cathode during the electrolysis of CuSO4 solution for 10 minutes with a current of 1.5 A is approximately 0.246 grams.

|
Explore Courses for Class 12 exam
|

|
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it?
Question Description
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it?.
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it?.
Solutions for Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? defined & explained in the simplest way possible. Besides giving the explanation of
Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it?, a detailed solution for Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? has been provided alongside types of Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? theory, EduRev gives you an
ample number of questions to practice Solution of cuso4 is electrolysed for 10 mins with a current of 1.5 A .what is the mass of 'cu' deposited at cathode? answer is w= 0.246 grams. can any one explain it? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

















