Class 12 Exam > Class 12 Questions > The correct statement(s) regarding hydrates o...
Start Learning for Free
The correct statement(s) regarding hydrates of aldehyde and ketone is/are
- a)Usually hydrates have lower thermodynamic stability than anhydrous form
- b)Hydrate content of acetone is greater in water than in hexane
- c)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
- d)C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
Correct answer is option 'A,B,C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The correct statement(s) regarding hydrates of aldehyde and ketone is/...
Hydrates of aldehydes and ketones are less stable than anhydrous form (gem diols are unstable).
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
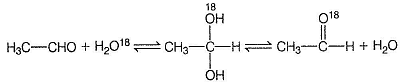
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
Most Upvoted Answer
The correct statement(s) regarding hydrates of aldehyde and ketone is/...
Hydrates of Aldehyde and Ketone:
1. Thermodynamic Stability:
- Usually, hydrates have lower thermodynamic stability than the anhydrous form.
- The presence of water in the structure of aldehydes and ketones affects their stability and reactivity.
- The formation of hydrates is an equilibrium process, and the stability of the hydrate depends on the balance between the hydration energy and the energy required to break the water-aldehyde or water-ketone interactions.
- In most cases, the anhydrous form is more stable than the corresponding hydrate.
2. Hydrate Content of Acetone:
- The hydrate content of acetone is greater in water than in hexane.
- Acetone (CH3COCH3) is a ketone that readily forms hydrates in the presence of water.
- In an aqueous environment, water molecules can interact with the carbonyl oxygen of acetone through hydrogen bonding, leading to the formation of hydrates.
- Hexane, on the other hand, is a non-polar solvent and does not readily form hydrates with acetone.
3. Isotopic Labeling:
- When CH3CHO (acetaldehyde) is treated with H2O18 (water containing the stable isotope oxygen-18) in acidic medium, it gets converted into CH3CHO18.
- This reaction is an example of isotopic labeling, where a specific atom or group of atoms is replaced with its isotopic counterpart.
- The acidic medium helps in the protonation of the carbonyl oxygen, making it more susceptible to nucleophilic attack by water molecules containing oxygen-18.
- The resulting product, CH3CHO18, contains the oxygen-18 isotope, which can be used for various studies and analyses.
4. Hydrate Content of C6H5CHO and p-nitrobenzaldehyde:
- C6H5CHO (benzaldehyde) and p-nitrobenzaldehyde both can form hydrates, but C6H5CHO has a greater hydrate content than p-nitrobenzaldehyde.
- The hydrate content depends on the ability of the aldehyde or ketone to form hydrogen bonds with water molecules.
- Benzaldehyde (C6H5CHO) has a phenyl group attached to the carbonyl carbon, which enhances its ability to form hydrogen bonds with water, leading to a higher hydrate content.
- In contrast, p-nitrobenzaldehyde has a nitro group (-NO2) attached to the carbonyl carbon, which reduces its ability to form hydrogen bonds with water, resulting in a lower hydrate content compared to benzaldehyde.
Therefore, the correct statements regarding hydrates of aldehyde and ketone are A) Usually hydrates have lower thermodynamic stability than the anhydrous form, B) Hydrate content of acetone is greater in water than in hexane, and C) CH3CHO when treated with H2O18 in acidic medium gets converted into CH3CHO18.
1. Thermodynamic Stability:
- Usually, hydrates have lower thermodynamic stability than the anhydrous form.
- The presence of water in the structure of aldehydes and ketones affects their stability and reactivity.
- The formation of hydrates is an equilibrium process, and the stability of the hydrate depends on the balance between the hydration energy and the energy required to break the water-aldehyde or water-ketone interactions.
- In most cases, the anhydrous form is more stable than the corresponding hydrate.
2. Hydrate Content of Acetone:
- The hydrate content of acetone is greater in water than in hexane.
- Acetone (CH3COCH3) is a ketone that readily forms hydrates in the presence of water.
- In an aqueous environment, water molecules can interact with the carbonyl oxygen of acetone through hydrogen bonding, leading to the formation of hydrates.
- Hexane, on the other hand, is a non-polar solvent and does not readily form hydrates with acetone.
3. Isotopic Labeling:
- When CH3CHO (acetaldehyde) is treated with H2O18 (water containing the stable isotope oxygen-18) in acidic medium, it gets converted into CH3CHO18.
- This reaction is an example of isotopic labeling, where a specific atom or group of atoms is replaced with its isotopic counterpart.
- The acidic medium helps in the protonation of the carbonyl oxygen, making it more susceptible to nucleophilic attack by water molecules containing oxygen-18.
- The resulting product, CH3CHO18, contains the oxygen-18 isotope, which can be used for various studies and analyses.
4. Hydrate Content of C6H5CHO and p-nitrobenzaldehyde:
- C6H5CHO (benzaldehyde) and p-nitrobenzaldehyde both can form hydrates, but C6H5CHO has a greater hydrate content than p-nitrobenzaldehyde.
- The hydrate content depends on the ability of the aldehyde or ketone to form hydrogen bonds with water molecules.
- Benzaldehyde (C6H5CHO) has a phenyl group attached to the carbonyl carbon, which enhances its ability to form hydrogen bonds with water, leading to a higher hydrate content.
- In contrast, p-nitrobenzaldehyde has a nitro group (-NO2) attached to the carbonyl carbon, which reduces its ability to form hydrogen bonds with water, resulting in a lower hydrate content compared to benzaldehyde.
Therefore, the correct statements regarding hydrates of aldehyde and ketone are A) Usually hydrates have lower thermodynamic stability than the anhydrous form, B) Hydrate content of acetone is greater in water than in hexane, and C) CH3CHO when treated with H2O18 in acidic medium gets converted into CH3CHO18.

|
Explore Courses for Class 12 exam
|

|
The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer?
Question Description
The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer?.
The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer?.
Solutions for The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer?, a detailed solution for The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? has been provided alongside types of The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The correct statement(s) regarding hydrates of aldehyde and ketone is/area)Usually hydrates have lower thermodynamic stability than anhydrous formb)Hydrate content of acetone is greater in water than in hexanec)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18d)C6H5CHO has greater hydrate content than p-nitrobenzaldehydCorrect answer is option 'A,B,C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
















