JEE Exam > JEE Questions > The French physicist Louis de-Broglie in 1924...
Start Learning for Free
The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.
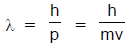
The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.
These waves differ from the electromagnetic waves as they,
(i) have lower velocities
(ii) have no electrical and magnetic fields and
(iii) are not emitted by the particle under consideration.
The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.
Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.
The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.
These waves differ from the electromagnetic waves as they,
(i) have lower velocities
(ii) have no electrical and magnetic fields and
(iii) are not emitted by the particle under consideration.
The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.
Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.
Q.
The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :
- a)2 → 5
- b)3 → 2
- c)2 → 6
- d)1 → 2
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The French physicist Louis de-Broglie in 1924 postulated that matter, ...
i.e. transition from
n = 1 to n = 3, n = 2 to n = 6
n = 3 to n = 9

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer?
Question Description
The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer?.
The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer?.
Solutions for The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer?, a detailed solution for The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The French physicist Louis de-Broglie in 1924 postulated that matter, like radiation, should exhibit a dual behaviour. He proposed the following relationship between the wavelength λ of a material particle, its linear momentum p and planck constant h.The de Broglie relation implies that the wavelength of a particle should decreases as its velocity increases. It also implies that for a given velocity heavier particles should have shorter wavelength than lighter particles. The waves associated with particles in motion are called matter waves or de Broglie waves.These waves differ from the electromagnetic waves as they,(i) have lower velocities(ii) have no electrical and magnetic fields and(iii) are not emitted by the particle under consideration.The experimental confirmation of the de–Broglie relation was obtained when Davisson and Germer, in 1927, observed that a beam of electrons is diffracted by a nickel crystal. As diffraction is a characteristic property of waves, hence the beam of electron behaves as a wave, as proposed by de–Broglie.Werner Heisenberg considered the limits of how precisely we can measure properties of an electron or other microscopic particle like electron. He determined that there is a fundamental limit of how closely we can measure both position and momentum. The more accurately we measure the momentum of a particle, the less accurately we can determine its position. The converse is also ture. This is summed up in what we now call the “Heisenberg uncertainty principle : It is impossible to determine simultaneously and precisely both the momentum and position of a particle. The product of undertainty in the position, Δx and the uncertainity in the momentum Δ(mv) must be greater than or equal to h/4π. i.e.Q. The transition, so that the de - Broglie wavelength of electron becomes 3 times of its initial value in He+ ion will be :a)2 → 5b)3 → 2c)2 → 6d)1 → 2Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























