Class 11 Exam > Class 11 Questions > Definition of diagonal relationship?
Start Learning for Free
Definition of diagonal relationship?
Verified Answer
Definition of diagonal relationship?
Diagonal Relationship
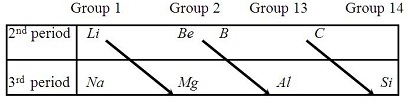
The similarity in the properties of denite pairs of diagonally adjacent elements in the second and third period periodic table is called diagonal relationship.
In s-block elements Lithium is the rst element of group 1 whereas Beryllium is the rst elementsof Group 2.
Some of their properties do not match with the properties exhibited by other elements of their group.
Instead their properties resemble the properties of the second element of the following group due to the simi ionic sizes and orcharge/radius ratio of the elements.
Consequently lithium and magnesium have similar properties whereas Beryllium and Aluminium exhibit simila properties.
This relation is called diagonal relationship.
 This question is part of UPSC exam. View all Class 11 courses
This question is part of UPSC exam. View all Class 11 courses
Most Upvoted Answer
Definition of diagonal relationship?
Diagonal relation ship is relation in properties of 2 and 3 period elements but it is valid till 4 group .The relationships are 1. Li - Mg2. Be - Al3. B - Sithere are only this three relationship in modern periodic table.
Community Answer
Definition of diagonal relationship?
Definition of Diagonal Relationship:
A diagonal relationship refers to the similarity in properties between two elements that are located diagonally from each other in the periodic table. These elements exhibit similar chemical behavior, despite belonging to different groups or periods. This phenomenon is observed in elements that are adjacent to each other but separated by a large number of elements in the periodic table.
Explanation:
Diagonal relationships occur between elements that have similar atomic sizes and valence electron configurations. These similarities lead to comparable chemical properties and reactivity. The most well-known example of diagonal relationships is observed between the elements lithium (Li) and magnesium (Mg), as well as between beryllium (Be) and aluminum (Al).
Key Points:
- Diagonal relationships exist between elements that are located diagonally from each other in the periodic table.
- Elements involved in diagonal relationships have similar properties and chemical behaviors.
- Diagonal relationships are observed between elements that have comparable atomic sizes and valence electron configurations.
- One of the most prominent examples of diagonal relationships is between lithium (Li) and magnesium (Mg), as well as between beryllium (Be) and aluminum (Al).
Characteristics of Diagonal Relationships:
Diagonal relationships share several characteristics that contribute to their similarities:
1. Atomic Size: Elements involved in diagonal relationships have similar atomic sizes due to their comparable number of electron shells. This similarity in size allows for similar chemical behavior and reactivity.
2. Valence Electron Configuration: Diagonal elements often have the same number of valence electrons, leading to comparable electronic configurations. This similarity in electron arrangement contributes to their similar chemical properties.
3. Oxidation States: Elements involved in diagonal relationships commonly exhibit similar oxidation states. For example, both lithium and magnesium typically have a +2 oxidation state, while beryllium and aluminum tend to have a +3 oxidation state.
4. Ionic Radii: Diagonal elements have similar ionic radii, which determines their ability to form ionic compounds. This similarity allows for the formation of compounds with similar crystal structures and properties.
Applications:
Understanding diagonal relationships is crucial in various fields, including chemistry and materials science. Some practical applications of diagonal relationships include:
- Predicting the properties and behavior of elements based on their position in the periodic table.
- Designing new materials with similar characteristics to known compounds.
- Developing catalysts and compounds with specific reactivity patterns based on diagonal relationships.
In conclusion, diagonal relationships refer to the similarity in properties between two elements located diagonally from each other in the periodic table. These relationships are based on similarities in atomic size, valence electron configuration, oxidation states, and ionic radii. Understanding diagonal relationships helps predict chemical behavior, design materials, and develop catalysts with specific characteristics.
A diagonal relationship refers to the similarity in properties between two elements that are located diagonally from each other in the periodic table. These elements exhibit similar chemical behavior, despite belonging to different groups or periods. This phenomenon is observed in elements that are adjacent to each other but separated by a large number of elements in the periodic table.
Explanation:
Diagonal relationships occur between elements that have similar atomic sizes and valence electron configurations. These similarities lead to comparable chemical properties and reactivity. The most well-known example of diagonal relationships is observed between the elements lithium (Li) and magnesium (Mg), as well as between beryllium (Be) and aluminum (Al).
Key Points:
- Diagonal relationships exist between elements that are located diagonally from each other in the periodic table.
- Elements involved in diagonal relationships have similar properties and chemical behaviors.
- Diagonal relationships are observed between elements that have comparable atomic sizes and valence electron configurations.
- One of the most prominent examples of diagonal relationships is between lithium (Li) and magnesium (Mg), as well as between beryllium (Be) and aluminum (Al).
Characteristics of Diagonal Relationships:
Diagonal relationships share several characteristics that contribute to their similarities:
1. Atomic Size: Elements involved in diagonal relationships have similar atomic sizes due to their comparable number of electron shells. This similarity in size allows for similar chemical behavior and reactivity.
2. Valence Electron Configuration: Diagonal elements often have the same number of valence electrons, leading to comparable electronic configurations. This similarity in electron arrangement contributes to their similar chemical properties.
3. Oxidation States: Elements involved in diagonal relationships commonly exhibit similar oxidation states. For example, both lithium and magnesium typically have a +2 oxidation state, while beryllium and aluminum tend to have a +3 oxidation state.
4. Ionic Radii: Diagonal elements have similar ionic radii, which determines their ability to form ionic compounds. This similarity allows for the formation of compounds with similar crystal structures and properties.
Applications:
Understanding diagonal relationships is crucial in various fields, including chemistry and materials science. Some practical applications of diagonal relationships include:
- Predicting the properties and behavior of elements based on their position in the periodic table.
- Designing new materials with similar characteristics to known compounds.
- Developing catalysts and compounds with specific reactivity patterns based on diagonal relationships.
In conclusion, diagonal relationships refer to the similarity in properties between two elements located diagonally from each other in the periodic table. These relationships are based on similarities in atomic size, valence electron configuration, oxidation states, and ionic radii. Understanding diagonal relationships helps predict chemical behavior, design materials, and develop catalysts with specific characteristics.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Definition of diagonal relationship?
Question Description
Definition of diagonal relationship? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Definition of diagonal relationship? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Definition of diagonal relationship?.
Definition of diagonal relationship? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Definition of diagonal relationship? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Definition of diagonal relationship?.
Solutions for Definition of diagonal relationship? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Definition of diagonal relationship? defined & explained in the simplest way possible. Besides giving the explanation of
Definition of diagonal relationship?, a detailed solution for Definition of diagonal relationship? has been provided alongside types of Definition of diagonal relationship? theory, EduRev gives you an
ample number of questions to practice Definition of diagonal relationship? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























