Class 12 Exam > Class 12 Questions > Which intermolecular force is present in Nylo...
Start Learning for Free
Which intermolecular force is present in Nylon 6,6?
- a)Copolymers
- b)Hydrogen bonding
- c)Dipole-dipole interaction
- d)van der Waals forces
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydro...
The correct answer is Option B.
Van der Waals forces include attraction and repulsions between atoms, molecules, and surfaces.
Hydrogen bonding is a special type of attraction between a hydrogen atom covalently bonded to a very electronegative atom such as N, O, or F atom and another very electronegative atom.
Dipole-dipole interactions occur when the partial charges formed within one molecule are attracted to an opposite partial charge in a nearby molecule.
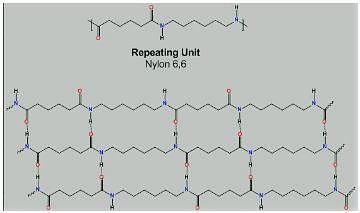
From the picture of Nylon-6,6, we can see there is hydrogen bonding between oxygen and hydrogen atom.
Though hydrogen bonds are also one type of dipole-dipole attraction, here, a more appropriate answer will be - The intermolecular forces present in nylon-6,6 are hydrogen bonding.
Hydrogen bonding is a special type of attraction between a hydrogen atom covalently bonded to a very electronegative atom such as N, O, or F atom and another very electronegative atom.
Dipole-dipole interactions occur when the partial charges formed within one molecule are attracted to an opposite partial charge in a nearby molecule.

From the picture of Nylon-6,6, we can see there is hydrogen bonding between oxygen and hydrogen atom.
Though hydrogen bonds are also one type of dipole-dipole attraction, here, a more appropriate answer will be - The intermolecular forces present in nylon-6,6 are hydrogen bonding.
Most Upvoted Answer
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydro...

Free Test
FREE
| Start Free Test |
Community Answer
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydro...
Introduction:
Nylon 6,6 is a synthetic polymer that contains repeating units of amide groups. It is composed of two monomers, adipic acid and hexamethylenediamine. The intermolecular forces present in Nylon 6,6 determine its physical and chemical properties, such as its melting point, solubility, and tensile strength. Among the various types of intermolecular forces, hydrogen bonding is the predominant force in Nylon 6,6.
Explanation:
Intermolecular Forces:
Intermolecular forces are the attractive forces that exist between molecules. These forces determine the physical properties of substances, such as boiling point, melting point, and solubility.
Hydrogen Bonding:
Hydrogen bonding is a type of intermolecular force that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and is attracted to another electronegative atom. In Nylon 6,6, the amide groups (-CONH-) contain a hydrogen atom bonded to the highly electronegative nitrogen atom. These hydrogen atoms can form hydrogen bonds with other electronegative atoms in neighboring molecules.
Presence of Hydrogen Bonding in Nylon 6,6:
In Nylon 6,6, the amide groups are present in the main chain of the polymer. The nitrogen atom in the amide group is highly electronegative and can form hydrogen bonds with the carbonyl oxygen atoms in neighboring amide groups. These hydrogen bonds contribute to the overall stability and strength of the polymer.
Effects of Hydrogen Bonding:
Hydrogen bonding in Nylon 6,6 leads to several important properties:
1. High Melting Point: Hydrogen bonding provides strong intermolecular attractions, resulting in a high melting point for Nylon 6,6. This makes it a suitable material for applications that require high-temperature stability.
2. High Tensile Strength: The hydrogen bonds between the amide groups contribute to the overall strength of Nylon 6,6. This polymer has excellent tensile strength, making it suitable for applications that require strong and durable materials.
3. Low Solubility: The presence of hydrogen bonding restricts the ability of Nylon 6,6 to dissolve in solvents. The hydrogen bonds between the amide groups are strong, and breaking these bonds requires a significant amount of energy.
Conclusion:
In conclusion, the intermolecular force that is present in Nylon 6,6 is hydrogen bonding. The presence of hydrogen bonds between the amide groups contributes to the high melting point, high tensile strength, and low solubility of Nylon 6,6. These properties make Nylon 6,6 a versatile and widely used polymer in various industrial applications.
Nylon 6,6 is a synthetic polymer that contains repeating units of amide groups. It is composed of two monomers, adipic acid and hexamethylenediamine. The intermolecular forces present in Nylon 6,6 determine its physical and chemical properties, such as its melting point, solubility, and tensile strength. Among the various types of intermolecular forces, hydrogen bonding is the predominant force in Nylon 6,6.
Explanation:
Intermolecular Forces:
Intermolecular forces are the attractive forces that exist between molecules. These forces determine the physical properties of substances, such as boiling point, melting point, and solubility.
Hydrogen Bonding:
Hydrogen bonding is a type of intermolecular force that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and is attracted to another electronegative atom. In Nylon 6,6, the amide groups (-CONH-) contain a hydrogen atom bonded to the highly electronegative nitrogen atom. These hydrogen atoms can form hydrogen bonds with other electronegative atoms in neighboring molecules.
Presence of Hydrogen Bonding in Nylon 6,6:
In Nylon 6,6, the amide groups are present in the main chain of the polymer. The nitrogen atom in the amide group is highly electronegative and can form hydrogen bonds with the carbonyl oxygen atoms in neighboring amide groups. These hydrogen bonds contribute to the overall stability and strength of the polymer.
Effects of Hydrogen Bonding:
Hydrogen bonding in Nylon 6,6 leads to several important properties:
1. High Melting Point: Hydrogen bonding provides strong intermolecular attractions, resulting in a high melting point for Nylon 6,6. This makes it a suitable material for applications that require high-temperature stability.
2. High Tensile Strength: The hydrogen bonds between the amide groups contribute to the overall strength of Nylon 6,6. This polymer has excellent tensile strength, making it suitable for applications that require strong and durable materials.
3. Low Solubility: The presence of hydrogen bonding restricts the ability of Nylon 6,6 to dissolve in solvents. The hydrogen bonds between the amide groups are strong, and breaking these bonds requires a significant amount of energy.
Conclusion:
In conclusion, the intermolecular force that is present in Nylon 6,6 is hydrogen bonding. The presence of hydrogen bonds between the amide groups contributes to the high melting point, high tensile strength, and low solubility of Nylon 6,6. These properties make Nylon 6,6 a versatile and widely used polymer in various industrial applications.

|
Explore Courses for Class 12 exam
|

|
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer?
Question Description
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer?.
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which intermolecular force is present in Nylon 6,6?a)Copolymersb)Hydrogen bondingc)Dipole-dipole interactiond)van der Waals forcesCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
















