Class 11 Exam > Class 11 Questions > Among the following substances, which one has...
Start Learning for Free
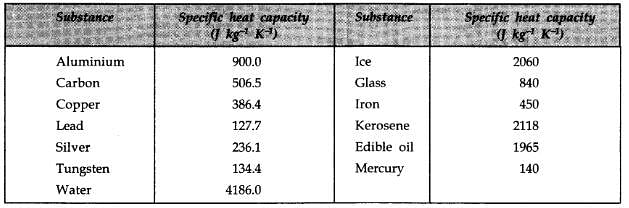
Among the following substances, which one has highest specific heat capacity?
- a)kerosene
- b)water
- c)Edible oil
- d)Ice
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Among the following substances, which one has highest specific heat ca...
Neutrinos would have the highest specific heat capacity if they qualify, because specific heat capacity is related to degrees of freedom per unit of mass, which is directly related to mass per particle (I can't call it atomic mass for particles smaller than H!), and their weight per particle is far, far lower than that of any element (or any other particle for that matter). However, neutrinos do not interact with photons and so can not absorb heat in the way normal substances do, so they probably don't qualify.The highest specific heat capacity is of the water
Therefore option (B) water is correct.
Most Upvoted Answer
Among the following substances, which one has highest specific heat ca...
Free Test
FREE
| Start Free Test |
Community Answer
Among the following substances, which one has highest specific heat ca...
Understanding Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. It is a crucial property in thermodynamics and helps in understanding how different substances respond to heat.
Comparison of Substances
- Kerosene: Has a specific heat capacity ranging from 1.9 to 2.1 J/g°C. It is reasonably efficient in heat storage but lower than water.
- Water: Water has a specific heat capacity of about 4.18 J/g°C, which is significantly higher than most substances. This high value allows water to absorb and retain heat effectively, making it essential for various biological and environmental processes.
- Edible Oil: Edible oils, such as olive or canola oil, typically have a specific heat capacity around 2.0 J/g°C, which is lower than that of water.
- Ice: Ice has a specific heat capacity of approximately 2.1 J/g°C. While it can store heat, it is still less effective than water in terms of heat capacity.
Conclusion
Among the options provided, water has the highest specific heat capacity. This property plays a vital role in regulating climate, supporting life, and influencing weather patterns. The ability of water to absorb large quantities of heat without a significant increase in temperature makes it an excellent coolant and a key component in many chemical processes.
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. It is a crucial property in thermodynamics and helps in understanding how different substances respond to heat.
Comparison of Substances
- Kerosene: Has a specific heat capacity ranging from 1.9 to 2.1 J/g°C. It is reasonably efficient in heat storage but lower than water.
- Water: Water has a specific heat capacity of about 4.18 J/g°C, which is significantly higher than most substances. This high value allows water to absorb and retain heat effectively, making it essential for various biological and environmental processes.
- Edible Oil: Edible oils, such as olive or canola oil, typically have a specific heat capacity around 2.0 J/g°C, which is lower than that of water.
- Ice: Ice has a specific heat capacity of approximately 2.1 J/g°C. While it can store heat, it is still less effective than water in terms of heat capacity.
Conclusion
Among the options provided, water has the highest specific heat capacity. This property plays a vital role in regulating climate, supporting life, and influencing weather patterns. The ability of water to absorb large quantities of heat without a significant increase in temperature makes it an excellent coolant and a key component in many chemical processes.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer?
Question Description
Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer?.
Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Among the following substances, which one has highest specific heat capacity?a)keroseneb)waterc)Edible oild)IceCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























