Class 8 Exam > Class 8 Questions > A strip of copper metal is given to you. desc...
Start Learning for Free
A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current.
Verified Answer
A strip of copper metal is given to you. describe briefly how you will...
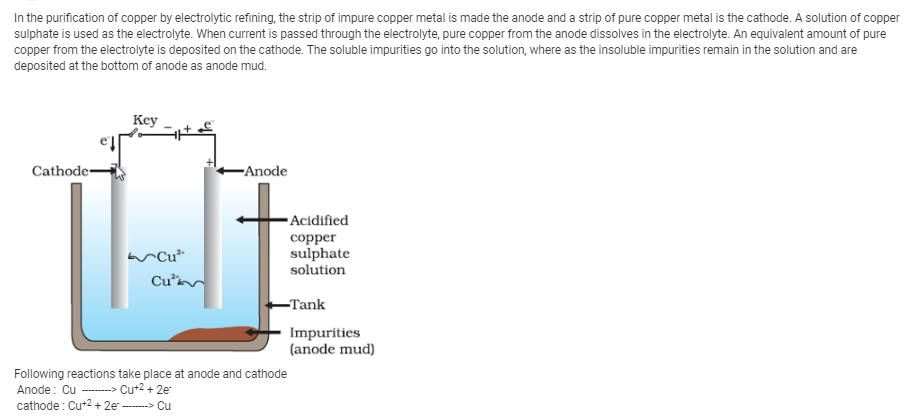
 This question is part of UPSC exam. View all Class 8 courses
This question is part of UPSC exam. View all Class 8 courses
Most Upvoted Answer
A strip of copper metal is given to you. describe briefly how you will...
Introduction:
To purify a strip of copper metal using the chemical effect of electric current, we can make use of a process called electrorefining. This process involves passing an electric current through a solution containing copper ions, which helps in removing impurities and obtaining pure copper.
Procedure:
1. Preparation of electrolyte: Prepare an electrolyte solution by dissolving a copper compound, such as copper sulfate (CuSO4), in water. This solution will allow the copper ions to move freely and participate in the electrorefining process.
2. Setting up the apparatus: Set up an electrolytic cell consisting of two electrodes – the impure copper strip as the anode (positive terminal) and a pure copper strip as the cathode (negative terminal). Ensure that the two electrodes are immersed in the electrolyte solution, but do not touch each other.
3. Passing electric current: Connect the anode and cathode to the terminals of a power supply, making sure that the anode is connected to the positive terminal and the cathode is connected to the negative terminal. This will complete the circuit and allow the electric current to flow through the electrolyte solution.
4. Electrolysis: When the electric current is passed through the electrolyte solution, copper atoms from the anode are oxidized and enter the solution as copper ions (Cu2+). These copper ions then migrate towards the cathode under the influence of the electric field.
5. Deposition: As the copper ions reach the cathode, they gain electrons and get reduced to form copper atoms. These copper atoms get deposited onto the cathode, gradually increasing its mass and purity. At the same time, impurities present in the anode do not get deposited on the cathode.
6. Collection of pure copper: After a certain period of time, the impure copper strip (anode) will become thinner, while the pure copper strip (cathode) will grow in size. The pure copper strip can be carefully removed, washed, and dried to obtain purified copper.
7. Repeating the process: If a higher level of purity is desired, the purified copper obtained from the previous step can be used as the anode in a new round of electrorefining. This process can be repeated multiple times to achieve a higher degree of purity.
Conclusion:
By utilizing the chemical effect of electric current in the process of electrorefining, we can purify a strip of copper metal and obtain high-purity copper. This method is widely used in industries to obtain pure copper for various applications.
To purify a strip of copper metal using the chemical effect of electric current, we can make use of a process called electrorefining. This process involves passing an electric current through a solution containing copper ions, which helps in removing impurities and obtaining pure copper.
Procedure:
1. Preparation of electrolyte: Prepare an electrolyte solution by dissolving a copper compound, such as copper sulfate (CuSO4), in water. This solution will allow the copper ions to move freely and participate in the electrorefining process.
2. Setting up the apparatus: Set up an electrolytic cell consisting of two electrodes – the impure copper strip as the anode (positive terminal) and a pure copper strip as the cathode (negative terminal). Ensure that the two electrodes are immersed in the electrolyte solution, but do not touch each other.
3. Passing electric current: Connect the anode and cathode to the terminals of a power supply, making sure that the anode is connected to the positive terminal and the cathode is connected to the negative terminal. This will complete the circuit and allow the electric current to flow through the electrolyte solution.
4. Electrolysis: When the electric current is passed through the electrolyte solution, copper atoms from the anode are oxidized and enter the solution as copper ions (Cu2+). These copper ions then migrate towards the cathode under the influence of the electric field.
5. Deposition: As the copper ions reach the cathode, they gain electrons and get reduced to form copper atoms. These copper atoms get deposited onto the cathode, gradually increasing its mass and purity. At the same time, impurities present in the anode do not get deposited on the cathode.
6. Collection of pure copper: After a certain period of time, the impure copper strip (anode) will become thinner, while the pure copper strip (cathode) will grow in size. The pure copper strip can be carefully removed, washed, and dried to obtain purified copper.
7. Repeating the process: If a higher level of purity is desired, the purified copper obtained from the previous step can be used as the anode in a new round of electrorefining. This process can be repeated multiple times to achieve a higher degree of purity.
Conclusion:
By utilizing the chemical effect of electric current in the process of electrorefining, we can purify a strip of copper metal and obtain high-purity copper. This method is widely used in industries to obtain pure copper for various applications.
Attention Class 8 Students!
To make sure you are not studying endlessly, EduRev has designed Class 8 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 8.

|
Explore Courses for Class 8 exam
|

|
Similar Class 8 Doubts
A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8
Question Description
A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 for Class 8 2024 is part of Class 8 preparation. The Question and answers have been prepared according to the Class 8 exam syllabus. Information about A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 covers all topics & solutions for Class 8 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8.
A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 for Class 8 2024 is part of Class 8 preparation. The Question and answers have been prepared according to the Class 8 exam syllabus. Information about A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 covers all topics & solutions for Class 8 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8.
Solutions for A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 in English & in Hindi are available as part of our courses for Class 8.
Download more important topics, notes, lectures and mock test series for Class 8 Exam by signing up for free.
Here you can find the meaning of A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 defined & explained in the simplest way possible. Besides giving the explanation of
A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8, a detailed solution for A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 has been provided alongside types of A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 theory, EduRev gives you an
ample number of questions to practice A strip of copper metal is given to you. describe briefly how you will purify it by using the chemical effect of electric current. Related: NCERT Solutions - Chemical Effects of Electric Current, Science, Class 8 tests, examples and also practice Class 8 tests.

|
Explore Courses for Class 8 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























