Chemistry Exam > Chemistry Questions > During titration of HCl and NH4OH the indicat...
Start Learning for Free
During titration of HCl and NH4OH the indicator can be used is/are:
- a)Methyl orange
- b)Phthalein phenol
- c)Litmus
- d)Methyl red
Correct answer is option 'A,C,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
During titration of HCl and NH4OH the indicator can be used is/are:a)M...


Most Upvoted Answer
During titration of HCl and NH4OH the indicator can be used is/are:a)M...
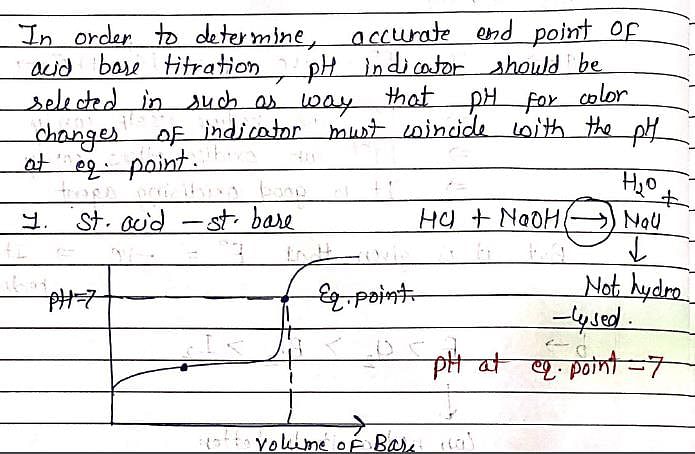
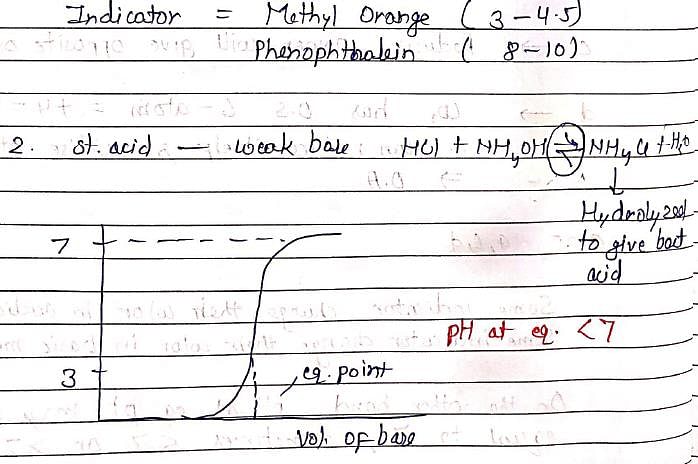
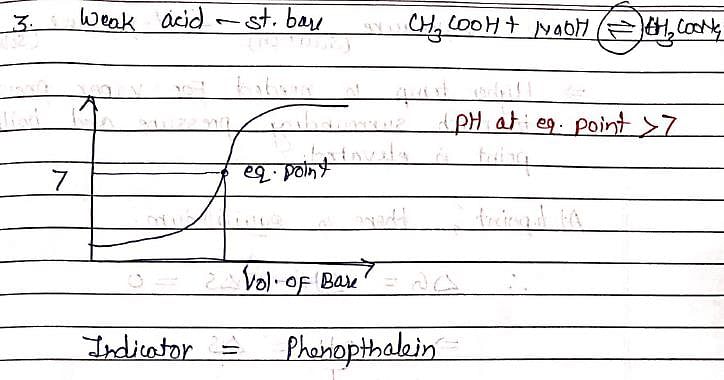
In the titration of HCl (strong acid) and NH4OH (weak base), except phenolphthalein all other indicators given in options are used.phenolphthalein is not used because:
let us suppose the condition in which HCl is taken in titration flask and NH4OH is taken in burette .since as we all know, phenolphthalein itself a weak acid indicator therefore when it added to HCl ,it increase its acidic strength.....so volume of NH4OH used to obtain end point is very high .however all other indicators such as methyl red,methyl orange,litmus ( ph range 5 -8)...are weakly basic in nature ...therefore decrease the acidic strength of hcl...so end point will be obtained easily...( volume of NH4OH taken in burette is enough to take reading)
let us suppose the condition in which HCl is taken in titration flask and NH4OH is taken in burette .since as we all know, phenolphthalein itself a weak acid indicator therefore when it added to HCl ,it increase its acidic strength.....so volume of NH4OH used to obtain end point is very high .however all other indicators such as methyl red,methyl orange,litmus ( ph range 5 -8)...are weakly basic in nature ...therefore decrease the acidic strength of hcl...so end point will be obtained easily...( volume of NH4OH taken in burette is enough to take reading)
Free Test
FREE
| Start Free Test |
Community Answer
During titration of HCl and NH4OH the indicator can be used is/are:a)M...
Indicator for titration of HCl and NH4OH
During the titration of HCl (hydrochloric acid) and NH4OH (ammonium hydroxide), the indicator can be used to determine the endpoint of the reaction. The indicator is a substance that undergoes a color change at a specific pH value, indicating the completion of the titration.
Indicators for the titration of HCl and NH4OH:
- Methyl orange: Methyl orange is a pH indicator that changes color in the pH range of 3.1 to 4.4. It is yellow in acidic solutions (pH < 3.1)="" and="" changes="" to="" red="" in="" basic="" solutions="" (ph="" /> 4.4). In the titration of HCl and NH4OH, methyl orange can be used to detect the endpoint when the solution changes from acidic (yellow) to slightly basic (red).
- Litmus: Litmus is a natural pH indicator that turns red in acidic solutions (pH < 7)="" and="" blue="" in="" basic="" solutions="" (ph="" /> 7). Although it does not have a specific pH range like methyl orange, it can still be used as an indicator in the titration of HCl and NH4OH to determine the endpoint when the solution reaches neutrality (pH 7).
- Methyl red: Methyl red is another pH indicator that changes color in the pH range of 4.2 to 6.3. It is red in acidic solutions (pH < 4.2)="" and="" changes="" to="" yellow="" in="" basic="" solutions="" (ph="" /> 6.3). In the titration of HCl and NH4OH, methyl red can be used to detect the endpoint when the solution changes from acidic (red) to slightly basic (yellow).
Explanation:
In the titration of HCl and NH4OH, the reaction between the two substances involves the neutralization of acid and base. HCl is a strong acid, while NH4OH is a weak base. The reaction can be represented by the following equation:
HCl + NH4OH → NH4Cl + H2O
During the titration, a solution of HCl is slowly added to a solution of NH4OH until the reaction is complete. The indicator is added to the solution as well. As the acid is added, the pH of the solution decreases, and the indicator will exhibit its characteristic color depending on the pH range it is sensitive to.
When the solution reaches the equivalence point, where all the acid has reacted with the base, the pH of the solution will change, and the indicator will undergo a color change. This color change indicates that the titration is complete, and the endpoint has been reached.
In the case of HCl and NH4OH titration, methyl orange, litmus, and methyl red can be used as indicators. Methyl orange is suitable for detecting the endpoint when the solution becomes slightly basic, litmus can be used to determine neutrality, and methyl red can be used to detect the endpoint when the solution becomes slightly acidic.
By using these

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer?
Question Description
During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer?.
During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer?.
Solutions for During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer?, a detailed solution for During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? has been provided alongside types of During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice During titration of HCl and NH4OH the indicator can be used is/are:a)Methyl orangeb)Phthalein phenolc)Litmusd)Methyl redCorrect answer is option 'A,C,D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















