Class 11 Exam > Class 11 Questions > When a chemical bond is formed, there is decr...
Start Learning for Free
When a chemical bond is formed, there is decrease in
- a)kinetic energy
- b)potential energy
- c)repulsive force
- d)attractive force
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
When a chemical bond is formed, there is decrease ina)kinetic energyb)...
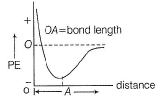
When two atoms approach to form a bond, there is decrease in potential energy (PE). After bond formation, at a minimum distance (called bond length). PE further increases.
Most Upvoted Answer
When a chemical bond is formed, there is decrease ina)kinetic energyb)...
When the chemical bond formed between atoms then new Product become stable.
stability is the inversely proportional to the potential energy.
stability is the inversely proportional to the potential energy.
Free Test
FREE
| Start Free Test |
Community Answer
When a chemical bond is formed, there is decrease ina)kinetic energyb)...
Explanation:
Chemical Bond Formation:
Chemical bond formation occurs when two or more atoms come close together and interact with each other by sharing or transferring electrons. This interaction leads to the formation of a stable compound.
Potential Energy:
Potential energy is the energy possessed by an object due to its position or composition. In the context of chemical bonds, potential energy refers to the energy stored in the arrangement of atoms within a molecule. It is the energy required to break the bonds and separate the atoms.
Decrease in Potential Energy:
When a chemical bond is formed, there is a decrease in potential energy. This can be understood by considering the forces acting between the atoms before and after bond formation.
- Repulsive Forces: Before bond formation, the electrons of one atom repel the electrons of another atom due to their negative charges. These repulsive forces try to separate the atoms.
- Attractive Forces: At the same time, there are attractive forces between the positively charged nucleus of one atom and the negatively charged electrons of another atom. These attractive forces try to bring the atoms closer together.
When the atoms come close enough, the attractive forces become stronger than the repulsive forces. The electrons rearrange and redistribute themselves to minimize the repulsion and maximize the attraction. This rearrangement leads to the formation of chemical bonds.
Stable Configuration:
The formation of a chemical bond allows the atoms to achieve a more stable configuration, where the potential energy is minimized. By sharing or transferring electrons, the atoms can achieve a filled outer electron shell, which is energetically favorable.
As a result, the potential energy of the system decreases when a chemical bond is formed. This decrease in potential energy is accompanied by the release of energy, which is often observed as heat or light.
Therefore, the correct answer is (b) potential energy.
Chemical Bond Formation:
Chemical bond formation occurs when two or more atoms come close together and interact with each other by sharing or transferring electrons. This interaction leads to the formation of a stable compound.
Potential Energy:
Potential energy is the energy possessed by an object due to its position or composition. In the context of chemical bonds, potential energy refers to the energy stored in the arrangement of atoms within a molecule. It is the energy required to break the bonds and separate the atoms.
Decrease in Potential Energy:
When a chemical bond is formed, there is a decrease in potential energy. This can be understood by considering the forces acting between the atoms before and after bond formation.
- Repulsive Forces: Before bond formation, the electrons of one atom repel the electrons of another atom due to their negative charges. These repulsive forces try to separate the atoms.
- Attractive Forces: At the same time, there are attractive forces between the positively charged nucleus of one atom and the negatively charged electrons of another atom. These attractive forces try to bring the atoms closer together.
When the atoms come close enough, the attractive forces become stronger than the repulsive forces. The electrons rearrange and redistribute themselves to minimize the repulsion and maximize the attraction. This rearrangement leads to the formation of chemical bonds.
Stable Configuration:
The formation of a chemical bond allows the atoms to achieve a more stable configuration, where the potential energy is minimized. By sharing or transferring electrons, the atoms can achieve a filled outer electron shell, which is energetically favorable.
As a result, the potential energy of the system decreases when a chemical bond is formed. This decrease in potential energy is accompanied by the release of energy, which is often observed as heat or light.
Therefore, the correct answer is (b) potential energy.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer?
Question Description
When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer?.
When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer?.
Solutions for When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When a chemical bond is formed, there is decrease ina)kinetic energyb)potential energyc)repulsive forced)attractive forceCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























