Class 12 Exam > Class 12 Questions > For an exothermic reaction, the energy of act...
Start Learning for Free
For an exothermic reaction, the energy of activation of the reactants is [1994]
- a)equal to the energy of activation of products
- b)less than the energy of activation of products
- c)greater than the energy of activation of products
- d)Sometimes greater and sometimes less than that of the products
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
For an exothermic reaction, the energy of activation of the reactants ...
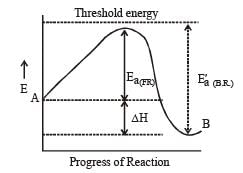
Ea(Forward) + ΔH = Ea(back word)
For Exothermic reaction, ΔH = –ve and
∴ activation energy of reactant is less than the energy of activation of products.
For Exothermic reaction, ΔH = –ve and
∴ activation energy of reactant is less than the energy of activation of products.
Most Upvoted Answer
For an exothermic reaction, the energy of activation of the reactants ...
Introduction:
In chemical reactions, the energy required to initiate the reaction is known as the energy of activation. It is the minimum amount of energy that the reactants must possess in order to undergo a chemical reaction. Exothermic reactions release energy in the form of heat, and in such reactions, the energy of activation of the reactants is less than the energy of activation of the products.
Explanation:
To understand why the energy of activation of the reactants is less than the energy of activation of the products in an exothermic reaction, let's consider the reaction profile diagram.
Reaction Profile Diagram:
A reaction profile diagram shows the energy changes that occur during a chemical reaction. It consists of an energy axis (y-axis) and the reaction progress (x-axis). The energy of the reactants is shown on the left side of the diagram, while the energy of the products is shown on the right side.
Key Points:
1. Energy of Activation of Reactants: The energy of activation of the reactants is the energy required to break the bonds in the reactant molecules and convert them into an unstable transition state. This energy is shown as the peak on the reaction profile diagram.
2. Energy of Activation of Products: The energy of activation of the products is the energy required to break the bonds in the transition state and convert it into the stable product molecules. This energy is also shown as a peak on the reaction profile diagram.
3. Exothermic Reaction: In an exothermic reaction, the energy released during the formation of new bonds in the products is greater than the energy required to break the bonds in the reactants. As a result, the overall energy change is negative, and the reaction releases heat.
4. Difference in Activation Energies: Since exothermic reactions release energy, the energy of activation of the products is higher than the energy of activation of the reactants. This is because the products have a lower energy level than the reactants, and it requires more energy to convert the transition state into stable products.
Conclusion:
In conclusion, the energy of activation of the reactants in an exothermic reaction is less than the energy of activation of the products. This is because exothermic reactions release energy, and the products have a lower energy level than the reactants. The energy of activation represents the minimum energy required to initiate a chemical reaction, and in exothermic reactions, this energy is lower for the reactants compared to the products.
In chemical reactions, the energy required to initiate the reaction is known as the energy of activation. It is the minimum amount of energy that the reactants must possess in order to undergo a chemical reaction. Exothermic reactions release energy in the form of heat, and in such reactions, the energy of activation of the reactants is less than the energy of activation of the products.
Explanation:
To understand why the energy of activation of the reactants is less than the energy of activation of the products in an exothermic reaction, let's consider the reaction profile diagram.
Reaction Profile Diagram:
A reaction profile diagram shows the energy changes that occur during a chemical reaction. It consists of an energy axis (y-axis) and the reaction progress (x-axis). The energy of the reactants is shown on the left side of the diagram, while the energy of the products is shown on the right side.
Key Points:
1. Energy of Activation of Reactants: The energy of activation of the reactants is the energy required to break the bonds in the reactant molecules and convert them into an unstable transition state. This energy is shown as the peak on the reaction profile diagram.
2. Energy of Activation of Products: The energy of activation of the products is the energy required to break the bonds in the transition state and convert it into the stable product molecules. This energy is also shown as a peak on the reaction profile diagram.
3. Exothermic Reaction: In an exothermic reaction, the energy released during the formation of new bonds in the products is greater than the energy required to break the bonds in the reactants. As a result, the overall energy change is negative, and the reaction releases heat.
4. Difference in Activation Energies: Since exothermic reactions release energy, the energy of activation of the products is higher than the energy of activation of the reactants. This is because the products have a lower energy level than the reactants, and it requires more energy to convert the transition state into stable products.
Conclusion:
In conclusion, the energy of activation of the reactants in an exothermic reaction is less than the energy of activation of the products. This is because exothermic reactions release energy, and the products have a lower energy level than the reactants. The energy of activation represents the minimum energy required to initiate a chemical reaction, and in exothermic reactions, this energy is lower for the reactants compared to the products.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer?
Question Description
For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer?.
For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer?.
Solutions for For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For an exothermic reaction, the energy of activation of the reactants is [1994]a)equal to the energy of activation of productsb)less than the energy of activation of productsc)greater than the energy of activation of productsd)Sometimes greater and sometimes less than that of the productsCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















