Class 9 Exam > Class 9 Questions > Difference between mixture and compound ?
Start Learning for Free
Difference between mixture and compound ?
Most Upvoted Answer
Difference between mixture and compound ?
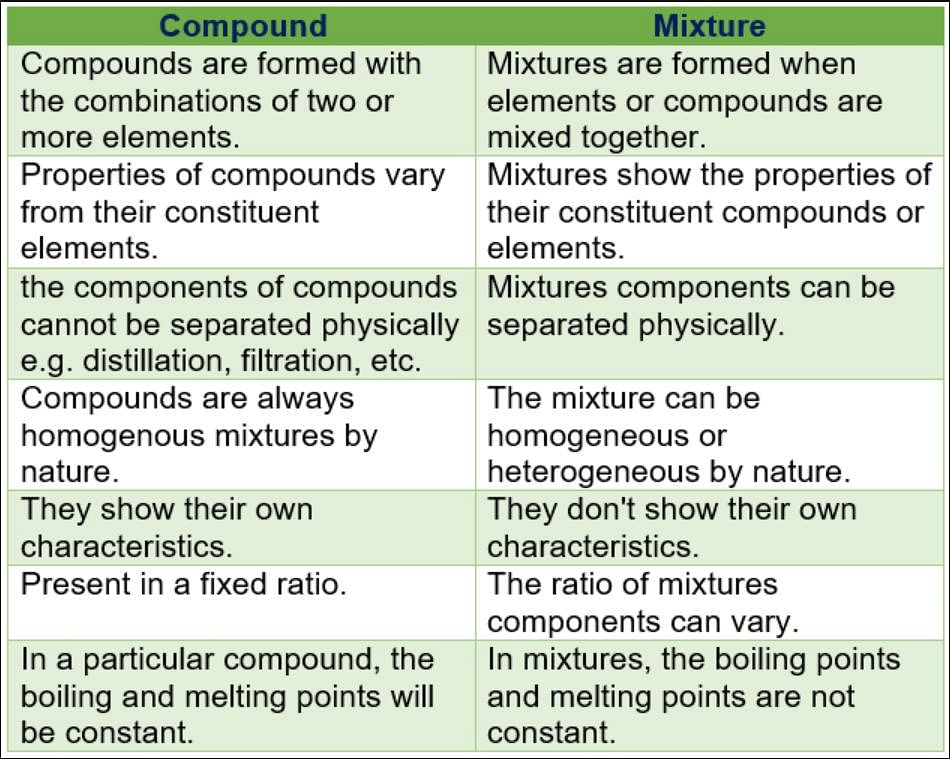
Community Answer
Difference between mixture and compound ?
Mixture:
A mixture is a combination of two or more substances that are physically combined and can be separated by physical means. In a mixture, the substances retain their individual properties and do not undergo any chemical changes. The components of a mixture can be present in any proportion and can be in different phases (solid, liquid, or gas).
Compound:
A compound, on the other hand, is a substance that is composed of two or more elements chemically bonded together in fixed proportions. Unlike mixtures, compounds have a definite chemical composition and exhibit unique properties different from the elements that make them up.
Key Differences:
Composition:
- Mixtures: Mixtures can have varying compositions where the proportion of components can be changed.
- Compounds: Compounds have a fixed composition with elements combined in specific ratios.
Physical Separation:
- Mixtures: Mixtures can be separated by physical means such as filtration, evaporation, or distillation.
- Compounds: Compounds cannot be separated by physical means but require chemical reactions to break the bonds between elements.
Properties:
- Mixtures: The properties of a mixture are the sum of the properties of its individual components. Each component retains its own properties.
- Compounds: Compounds have unique properties different from the elements that form them. They often exhibit new properties that are not observed in the individual elements.
Chemical Composition:
- Mixtures: In mixtures, the substances are physically combined and can exist in different phases.
- Compounds: Compounds are chemically combined, and the elements lose their individual identities to form a new substance with different properties.
Examples:
- Mixtures: Examples of mixtures include air (a mixture of gases), saltwater (a mixture of salt and water), and trail mix (a mixture of nuts, seeds, and dried fruits).
- Compounds: Examples of compounds include water (H2O), carbon dioxide (CO2), and sodium chloride (NaCl).
Summary:
In summary, mixtures are combinations of substances that can be separated by physical means and retain the properties of their individual components. Compounds, on the other hand, are substances formed by the chemical combination of elements in fixed proportions and exhibit unique properties. While mixtures have variable compositions and can exist in different phases, compounds have fixed compositions and require chemical reactions for separation.
A mixture is a combination of two or more substances that are physically combined and can be separated by physical means. In a mixture, the substances retain their individual properties and do not undergo any chemical changes. The components of a mixture can be present in any proportion and can be in different phases (solid, liquid, or gas).
Compound:
A compound, on the other hand, is a substance that is composed of two or more elements chemically bonded together in fixed proportions. Unlike mixtures, compounds have a definite chemical composition and exhibit unique properties different from the elements that make them up.
Key Differences:
Composition:
- Mixtures: Mixtures can have varying compositions where the proportion of components can be changed.
- Compounds: Compounds have a fixed composition with elements combined in specific ratios.
Physical Separation:
- Mixtures: Mixtures can be separated by physical means such as filtration, evaporation, or distillation.
- Compounds: Compounds cannot be separated by physical means but require chemical reactions to break the bonds between elements.
Properties:
- Mixtures: The properties of a mixture are the sum of the properties of its individual components. Each component retains its own properties.
- Compounds: Compounds have unique properties different from the elements that form them. They often exhibit new properties that are not observed in the individual elements.
Chemical Composition:
- Mixtures: In mixtures, the substances are physically combined and can exist in different phases.
- Compounds: Compounds are chemically combined, and the elements lose their individual identities to form a new substance with different properties.
Examples:
- Mixtures: Examples of mixtures include air (a mixture of gases), saltwater (a mixture of salt and water), and trail mix (a mixture of nuts, seeds, and dried fruits).
- Compounds: Examples of compounds include water (H2O), carbon dioxide (CO2), and sodium chloride (NaCl).
Summary:
In summary, mixtures are combinations of substances that can be separated by physical means and retain the properties of their individual components. Compounds, on the other hand, are substances formed by the chemical combination of elements in fixed proportions and exhibit unique properties. While mixtures have variable compositions and can exist in different phases, compounds have fixed compositions and require chemical reactions for separation.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
Difference between mixture and compound ?
Question Description
Difference between mixture and compound ? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Difference between mixture and compound ? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between mixture and compound ?.
Difference between mixture and compound ? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Difference between mixture and compound ? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between mixture and compound ?.
Solutions for Difference between mixture and compound ? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of Difference between mixture and compound ? defined & explained in the simplest way possible. Besides giving the explanation of
Difference between mixture and compound ?, a detailed solution for Difference between mixture and compound ? has been provided alongside types of Difference between mixture and compound ? theory, EduRev gives you an
ample number of questions to practice Difference between mixture and compound ? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























