Acidity & Basicity of Organic Compounds | Chemistry for JEE Main & Advanced PDF Download
Arrhenius theory of Acids & Bases
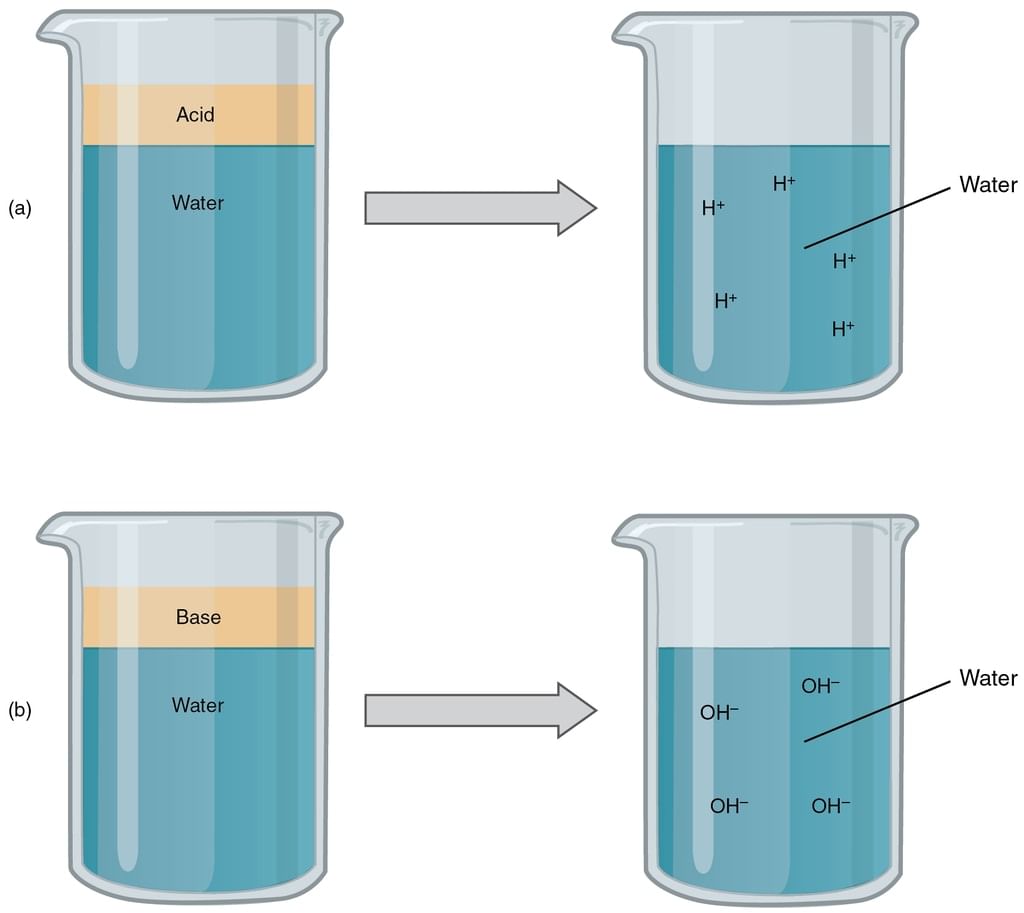
According to Arrhenius concept of acid and bases,
1. Acid
- An acid is a substance that releases H+ ions on dissolving in water.
- Greater the number of H+ ions produced in the solution, the stronger is the acid.
2. Bases
- A base is a substance that releases OH−ions on dissolving in water.
- Similarly greater the number of OH−ions produced in the aqueous solution, the stronger is the base.
Dissociation of Acids & Bases
Dissociation of weak electrolytes, (of weak acids and weak bases)
Weak electrolytes are ionized to only a slight extent, the concentration of ions are relatively low; most solute molecules do not split into ions.
The extent to which the weak electrolytes dissociates is expressed by either ionization constants or degree of ionization.
A weak monoprotic acid
HA + H2O ⇄ H3O+ + A-
A weak base
B + H2O ⇄ BH+ + OH-
Equilibrium constants of ionization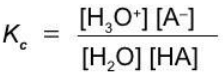
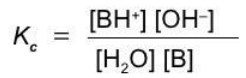
Acid and base ionization constants
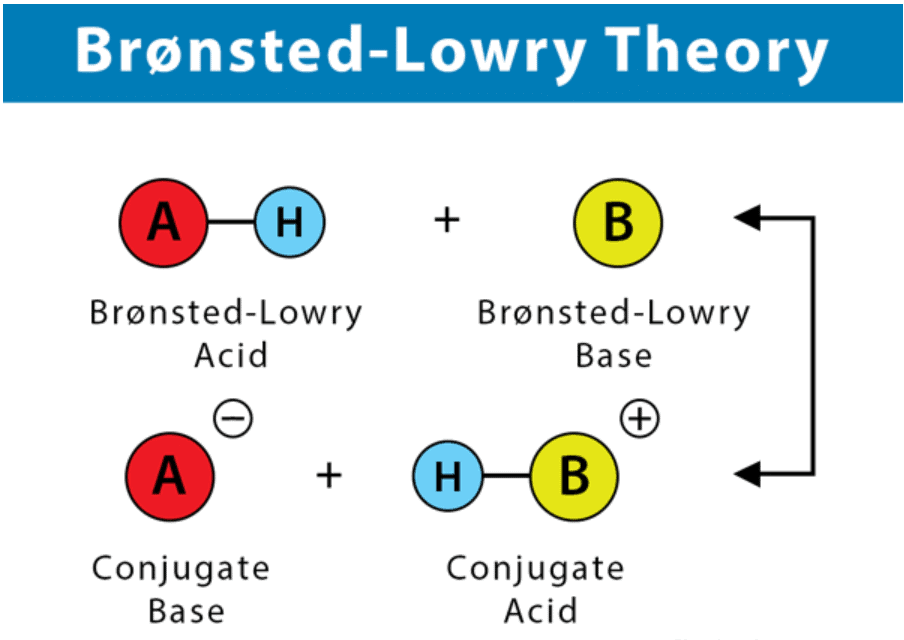
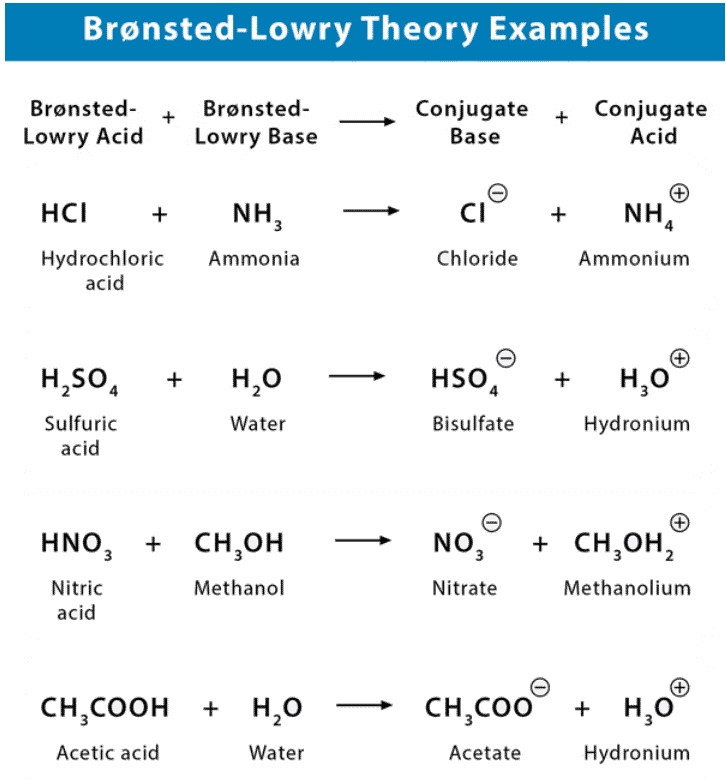
Bronsted Lowry Theory of Acids and Bases
- A Brønsted-Lowry acid must contain at least one hydrogen atom, typically attached to a very electronegative atom such as oxygen.
- When a Brønsted-Lowry acid donates a proton, it loses one H from its molecular formula, and its charge decreases by one.
- The resulting species is a base since the reversal of the loss of proton is the gain of a proton.
- The species gaining the proton is acting as a proton acceptor or a Brønsted-Lowry base.
Furthermore, when an acidic substance loses a proton, it forms a base, called the conjugate base of the acid. When a basic substance gains a proton, it forms an acid called the conjugate acid of a base.
The strength of a base is measured by its ability to capture protons. In contrast, the strength of an acid is measured by its ability to donate protons.
Therefore, the stronger the acid, the weaker the conjugate base, and the stronger the base, the weaker is its conjugate acid.
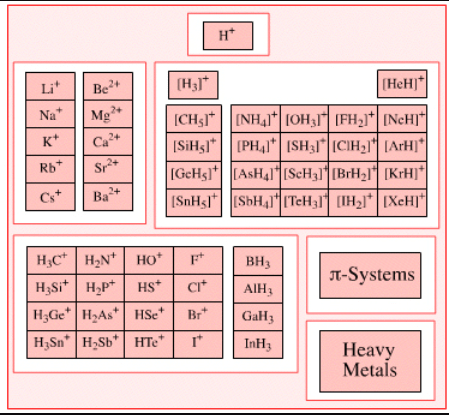
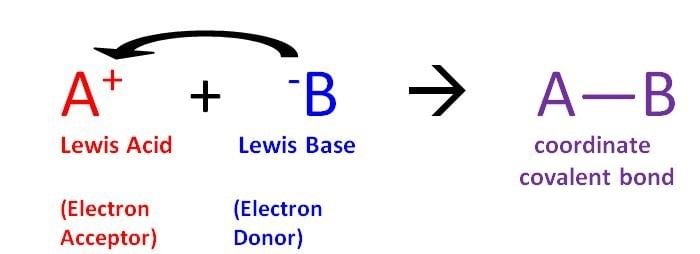
Lewis Theory of Acid & Bases
Lewis Acid
- Lewis Acids are the chemical species that have empty orbitals and are able to accept electron pairs from Lewis bases.
- This term was classically used to describe chemical species with a trigonal planar structure and an empty p-orbital. An example of such a Lewis acid would be BR3 (where R can be a halide or an organic substituent).
- Water and some other compounds are considered as both Lewis acids and bases since they can accept and donate electron pairs based on the reaction.
Examples of Lewis Acids
Some common examples of Lewis acids that can accept electron pairs include:
- H+ ions (or protons) can be considered as Lewis acids along with onium ions like H3O+.
- The cations of d block elements that display high oxidation states can act as electron-pair acceptors. An example of such a cation is Fe3+.
- Cations of metals such as Mg2+ and Li+ can form coordination compounds with water acting as the ligand. These aqua complexes can accept electron pairs and behave as Lewis acids.

- Carbocations given by H3C+ and other trigonal planar species tend to accept electron pairs.
- The Pentahalides of the following group 15 elements can act as Lewis acids – Antimony, Arsenic, and Phosphorus.
- Apart from these chemical compounds listed above, any electron-deficient π system can act as an acceptor of electron pairs – enones, for example.
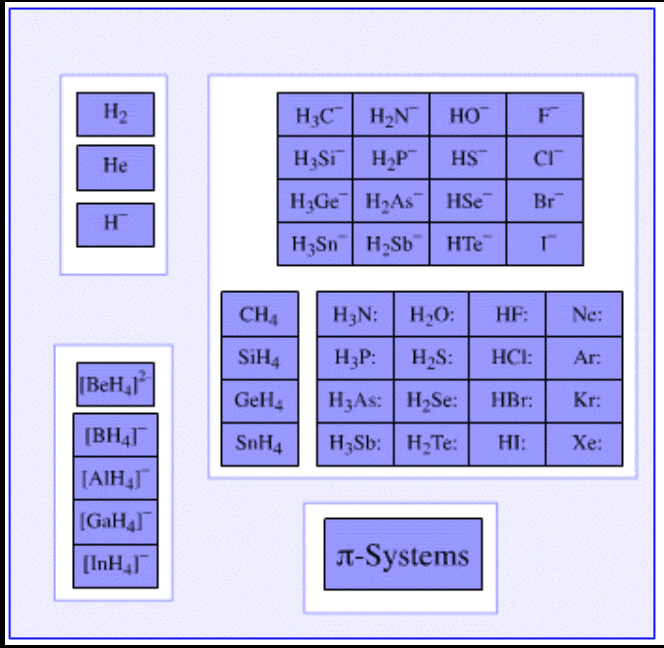
Lewis Base
- Atomic or molecular chemical species having a highly localized HOMO (The Highest Occupied Molecular Orbital) act as Lewis bases.
- These chemical species have the ability to donate an electron pair to a given Lewis acid in order to form an adduct, as discussed earlier.
- The most common Lewis bases are ammonia, alkyl amines, and other conventional amines.
- Commonly, Lewis bases are anionic in nature and their base strength generally depends on the pKa of the corresponding parent acid.
- Since Lewis bases are electron-rich species that have the ability to donate electron-pairs, they can be classified as nucleophiles.
- Similarly, Lewis acids can be classified as electrophiles (since they behave as electron-pair acceptors).
Examples of Lewis Bases
- Examples of Lewis bases which have an ability to donate an electron pair are listed below.
- Pyridine and the derivatives of pyridine have the ability to act as electron pair donors. Thus, these compounds can be classified as Lewis bases.
- The compounds in which Oxygen, Sulphur, Selenium, and Tellurium (which belong to group 16 of the Periodic Table) exhibit an oxidation state of -2 are generally Lewis bases.
- Examples of such compounds include water and ketones.

- The simple anions which have an electron pair can also act as Lewis bases by donating these electrons. Examples of such anions include H– and F–. Even some complex anions, such as the sulfate anion (SO42-) can donate pairs of electrons.
- The π-systems which are rich in electrons (such as benzene, ethyne, and ethene) exhibit great electron pair donating capabilities.
- Weak Lewis acids have strong conjugate Lewis bases.
- Apart from this, many chemical species having a lone pair of electrons such as CH3– and OH– are identified as Lewis bases due to their electron pair donating capabilities.
Ex.1 Compare the acidic strength of the following acids.
(a) C - C - C - COOH (b) C = C - C - COOH (c) C≡C-C-COOH
Sol. The acid whose conjugate base is most stable will be more acidic.
After forming conjugate base from the above acids.
(a) 
(b) 
(c) 
It is clear that sp hybridised carbon being most electronegative will decrease e-density from O most effectively making the conjugate base most stable.
c > b > a (acidic strength)
Ex.2 Which is more acidic between the two:
(a) CHF3 (b) CHCl3
Sol. CHF3 > CHCl3
If we consider the -I effect of F and Cl But this effect will not be considered here
After the removal of proton
(a)  (b)
(b) 
(vacant d-orbital available where C will coordinate its electron) (pπ - dπ bonding)
→ a < b (acidic strength)
Ex.3 Compare the acidic strength of the following:
(a) CHF3 (b) CHCl3
(c) CHBr3
(pπ - dπ bonding in Br is not as much as effective as in Cl due to large size of Br)
Sol. CHCl3 > CHBr3 > CHF3
Ex.4 Compare the acidic strength of the following
(a) CH (CN)3 (b) CH (NO2)3 (c) CHCl3
Sol. After removing H
 (Resonance) In its resonating structure, -ve charge will be on N)
(Resonance) In its resonating structure, -ve charge will be on N)
 (Resonance) (- In its resonating structure -ve charge will reside on O)
(Resonance) (- In its resonating structure -ve charge will reside on O)
→ more effective Resonance
 (pπ - dπ)
(pπ - dπ)
b > a > c
* -ve charge on O is more
stable than -ve charge on N as O is more electronegative than N.
* pπ - dπ Resonance < Actual Resonance
Ex.5 Compare the acidic strength of the following:
(a) CH≡CH (b) CH2 = CH2 (c) CH3 - CH3
Sol. 
(Stability of the conjugate base)
→ a > b > c (acidic strength)
Ex.6 Compare the acidic strength of the following :
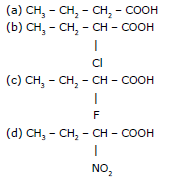
Sol. d > c > b > a
Ex.7 Compare the acidic strength of the following :
(a) H2O (b) H2S (c) H2Se (d) H2Te
Sol. Conjugate base has the stability order:

→ H2O < H2S < H2Se < H2Te (acidic strength)
Ex.8 Compare the acidic strength of the following compound:
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Sol. After forming conjugate base of the above:



c > d > b > a
Ex.9 Compare the reactivity of the following compounds with 1 mole of AgNO3
(a) 
(b) 
(c) 
(d) 
Sol. After removing Cl-


( ve charge is not on resonance least stable)

(most stable as L.P. of Cl will be coordinated to ve charge completing the octet of each atom and making the carbocation most stable)

extent of +ve charge decreases stability increases.
Ex.10 Compare the acidic strength:
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Sol. After making conjugate base




c > b > a > d
Basic Strength

Basic strength directly depends on the availability of lone pair for H.
Ex.11 Compare the basic strength of following:
Sol. 
Ex.12 Compare the basic strength of the following
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Sol.  ,
,  ,
,  ,
, 
CH4 < NH3 < H2O < HF
(acidic strength)

* Strong Acids have weak conjugate base.

* For the same period
less electronegativity, more nucleophilicity as more electronegative element has less tendency to give its electron pair.
Ex.13 Which is more basic  or
or  ?
?
Sol.  >
> 
Which is more basic NH3 or
forming conjugate acid


Comparison of Basicity of Ammonia and Alkyl Amines
Ex.14 Compare the basic strength of the following NH3, CH3NH2, (CH3)2NH, (CH3)3N
Factors which affect the basicity of Amines
(1) steric effect (2) Inductive effect (3) solvation effect.
The base whose conjugate acid is more stable will be more acidic forming conjugate acid of the given base.

Stability order of conjugate acid

Therefore basic strength,
(CH3)3N > (CH3)2NH > CH3NH2 > NH3
(vapor phase or gaseous phase or in Non polar solvent)
In Aqueous solution or in polar solvent
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
In aqueous solution, the conjugate acids form H-bonds (intermolecular) with water molecules and stabilise themselves. Conjugate acid of 1° amine which has largest no. of H-atoms form maximum H-bond with water and is most stable. Consequently 1° amine is most basic.
Due to steric effect 1° amine is considered more basic as compared to 3° amine as lone pair is hindered by three alkyl group and less available for H .
Considering the combined effect of the three (Inductive, solvation and steric effect) we can conclude that
2° > 1° > 3° > NH3
Aromatic amines are least basic as their lone pair is in conjugation and less available for protonation.
Ex.15 Compare the basic strength of the following:
(a)

(b) 
(c) 
(if L.P. will participate in Resonance, then molecule becomes aromatic)
Hence L.P. will have a greater tendency to take part in Resonance and will be less available for H+
This compound will be least basic.
Ex.16 Compare the basic strength of the following:

Sol. sp hybridised carbon being most electronegative will attract e- density from nitrogen and will make it less available for H+. Hence basicity decreases.
c > b > a
Ex.17 Compare the basic strength:
(a) 
(b) 
a < b
Ex.18 Compare the basicity of the following compounds:
(a) CH3 - CH2 - CH = CH - 
(b) 
(c) 
(d) 
Sol. In part (a) the lone pair of nitrogen is in resonance therefore will be less available for H making it least basic among all followed by sp, sp2, sp3 hybridised carbon atoms.
b > c > d > a
Ex.19 Compare the basicity of the numbered nitrogen atoms.

Sol. The planarity of ring will be destroyed if L.P. will take part in Resonance.
Basicity order of Nitrogen follows the order:
N(sp3) > N(sp2) > N(sp)

(In this sp2, l.p. is in Resonance with ring hence will be less available for H+ therefore it will be least basic)
Ex.20 Compare the basic strength of the following:
(a) 
(b) 
(c) 
Sol. In part (a) NO2 is at p-position Hence will attract e- density by both -M and -I.
In part (b) NO2 is at m-position hence will attract e- density by -I only
There is no such effect in part (c)
→ Availability of L.P. on nitrogen in part (a) is minimum followed by b and then c.
c > b > a
Ortho effect :
The ortho substituted aniline are less basic than aniline and ortho substituted benzoic acids are more acidic than benzoic acid.
Ortho effect is valid only for benzoic acid and aniline.
e.g. 
Also 
Ex.21 Compare the basic strength of the following :
(a) 
(b) 
(c) 
(d) 
Sol. a > b > d > c
* Due to ortho effect d > c
if c is less basic than d then it will be certainly less basic than b as b is more basic than d.
Ex.22 Compare the basic strength of the following :
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Sol. Do yourselves.
S.I.P → Steric inhibition of Protonation (ortho effect)

After protonation, repulsion increases therefore ortho substituted aniline is less basic than aniline.
S.I.R → Steric inhibition of resonance
(a) 
(b) 
|
352 videos|596 docs|309 tests
|
FAQs on Acidity & Basicity of Organic Compounds - Chemistry for JEE Main & Advanced
| 1. What is the Arrhenius theory of acids and bases? |  |
| 2. What is the Bronsted-Lowry theory of acids and bases? |  |
| 3. What is the Lewis theory of acids and bases? |  |
| 4. What is a Lewis base? |  |
| 5. Can you provide examples of Lewis bases? |  |
| 6. How does the basicity of ammonia compare to alkyl amines? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|