Acids and Bases | Chemistry for ACT PDF Download
Introduction
The earliest criteria for the characterization of acids and bases were the experimentally observed properties of aqueous solutions.
- An acid was defined as a substance whose water solution tastes sour, turns blue litmus red, neutralizes bases and so on.
- A substance was a base if its aqueous solution tasted bitter, turns red litmus blue, neutralizes acids and so on.

Faraday termed acids, bases and salts as electrolytes and Liebig proposed that acids are compounds containing hydrogen that metals can replace. Different investigators have put forth different concepts to characterize acids and bases but the following are the three important modern concepts of acids and bases:
Arrhenius Concept
According to Arrhenius's concept all substances which give H+ ions when dissolved in water are called acids, while those which ionize in water to furnish OH- ions are called bases.
HA ⇌ H+ + A- (Acid)
BOH ⇌ B+ + OH- (Base)
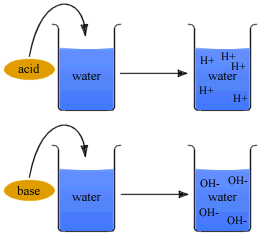
Thus, HCl is an acid because it gives H+ ions in water. Similarly, NaOH is a base as it yields OH- ions in water.
HCl ⇌ H+ + Cl-
NaOH ⇌ Na+ + OH-
- Some acids and bases ionize completely in solutions and are called string acids and bases.
- Others are dissociated to a limited extent in solutions and are termed weak acids and bases.
- Strong Acids: HCl, HNO3, H2SO4, HCIO4
- Strong Bases: NaOH, KOH, (CH3)4NOH
Every hydrogen compound cannot be regarded as an acid, e.g., CH4 is not an acid. Similarly, CH3OH, C2H5OH, etc., have OH groups but they are not bases, they must generate H+ or OH- ions in aqueous solution in order to be defined as acid or base.
Actually free H+ ions are highly reaction so they do not exist in water. They combine with water molecules to form hydronium ion (H3O+) molecules, i.e., have strong tendency to get hydrated.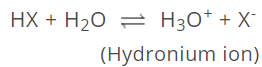
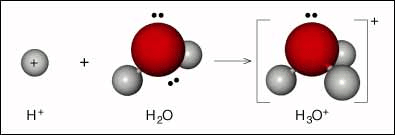
The proton in aqueous solution is generally represented as H+ (aq). It is now known that almost all the ions are hydrated to more or less extent and it is customary to put (aq) after each ion.
Acidic Oxide: The oxides of many non-metals react with water to form acids and are called acidic oxides or acid anhydrides.
CO2 + H2O H2CO3 ⇌ 2H+(aq) + (aq)
N2O5 + H2O 2HNO3 ⇌ 2H+(aq) + (aq)
Basic Oxide: Many oxides of metals dissolve in water to form hydroxides. Such oxides are termed basic oxides.
Na2O + H2O → 2NaOH ⇌ 2Na+(aq) + 2OH- (aq)
The substance like NH3 and N2H4 act as bases as they react with water to produce OH- ions.
NH3 + H2O → NH4OH ⇌ NH+4 (aq) + OH- (aq)
Neutralization Reaction: The reaction between an acid and a base is termed neutralization. According to the Arrhenius concept, the neutralization in aqueous solution involves the reaction between H+ and OH- ions or hydronium and OH-. This can be represented as
H3O+ + OH- ⇌ 2H2O
Limitations of Arrhenius Concept
- For the acidic or basic properties, the presence of water is absolutely necessary. Dry HCl shall not act as an acid. HCl is regarded as an acid only when dissolved in water and not in any other solvent.
- The concept does not explain acidic and basic character of substances in non-aqueous solvents.
- The neutralization process is limited to those reactions which can occur in aqueous solutions only, although reactions involving salt formation do occur in the absence of solvent.
- It cannot explain the acidic character of certain salts such as AlCl3 in aqueous solution.
- An artificial explanation is required to explain the basic nature of NH3 and metallic oxides and acidic nature of non-metal oxides.
Bronsted-Lowry Concept - The Proton-Donor-Acceptor Concept
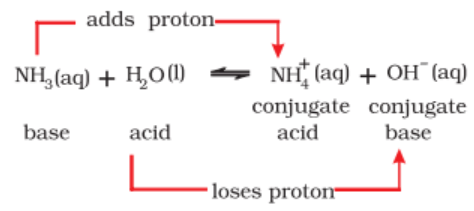
In 1923, Bronsted and Lowry independently proposed a broader concept of acids and bases. According to Bronsted-Lowry concept an acid is a substance (molecule or ion) that can donate proton, i.e., a hydrogen ion, H+, to some other substance and a base is a substance that can accept a proton from an acid. More simply, an acid is a proton donor (protogenic) and a base is a proton acceptor (protophilic).
Consider the reaction,
HCl + H2O ⇌ H3O + Cl-
In this reaction HCl acts as an acid because it donates a proton to the water molecule. Water, on the other hand, behaves as a base by accepting a proton from the acid.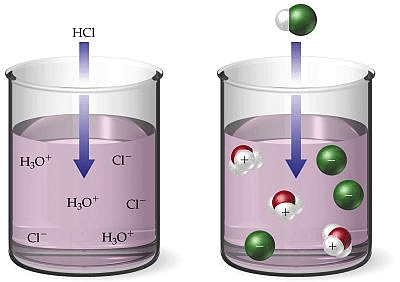
The dissolution of ammonia in water may be represented as
NH3 + H2O ⇌ NH4+ + OH-
In this, reaction, H2O acts as an acid it donated a proton to NH3 molecule and NH3molecule behaves as a base as it accepts a proton.
When an acid loses a proton, the residual part of it has a tendency to regain a proton. Therefore, it behaves as a base.
Acid ⇌ H+ + Base
The acid and base which differ by a proton are known to form a conjugate pair. Consider the following reaction.
CH3COOH + H2O ⇌ H3O+ + CH3COO-
It involves two conjugate pairs. The acid-base pairs are: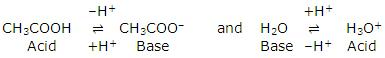
Such pairs of substances that can be formed from one another by the loss or gain of a proton are known as conjugate acid-base pairs.
If in the above reaction, the acid CH3COOH is labelled acid1 and its conjugate base, CH3COO- as base1. H2O is labelled as base2 and its conjugate acid H3O+ as acid2, the reaction can be written as:
Acid1 + Base2 ⇌ Base1 + Acid2
Thus, any acid-base reaction involves two conjugate pairs, i.e., when an acid reacts with a base, another acid and base are formed.
Thus, every acid has its conjugate base and every base has its conjugate acid. It is further observed that strong acids have weak conjugate bases while weak acids have strong conjugate bases.
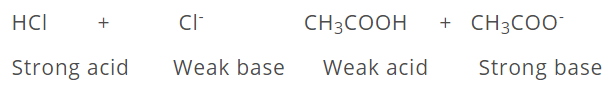
There are certain molecules which have dual character of an acid and a base. These are called amphiprotic or atmospheric.
Examples are NH3, H2O, CH3COOH, etc.
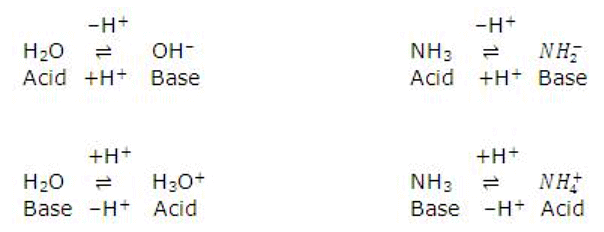
The strength of an acid depends upon its tendency to lose its proton and the strength of the base depends upon its tendency to gain the proton. 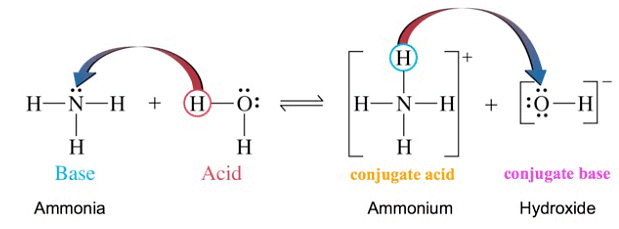
An acid-Base chart containing some common conjugate acid-base pairs
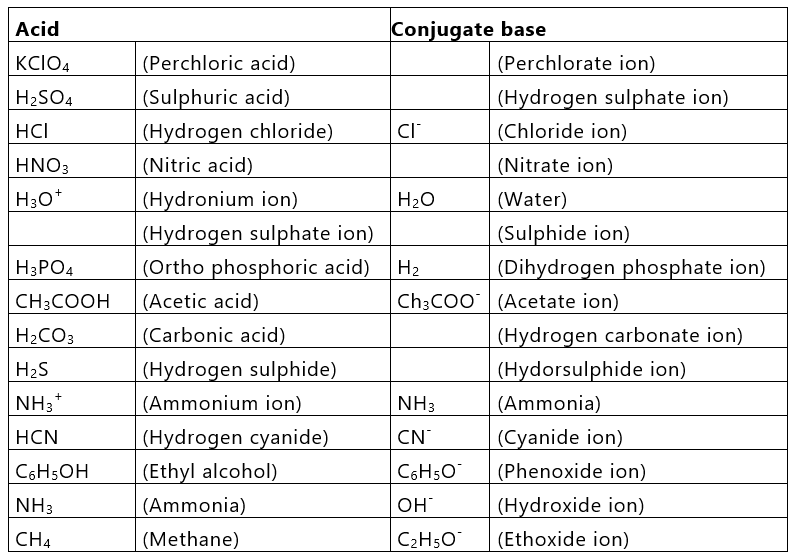
- In acid-base strength series, all acids above H3O+ in aqueous solution fall to the strength of H3O+.
- Similarly, the basic strength of bases below OH- fall to the strength of OH- in aqueous solution. This is known as leveling effect.
- The strength of an acid also depends upon the solvent.
- The acids HCIO4, H2S04, HCl and HN03 which have nearly the same strength in water will be in the order of HClO4> H2SO4 > HCl > HNO3 in acetic acid, since the proton accepting tendency of acetic acid is much weaker than water.
- So the real strength of acids can be judged by solvents.
On the basis of proton interaction, solvents can be classified into four types:
- Protophilic solvents: Solvents which have greater tendency to accept protons, i.e., water, alcohol, liquid ammonia, etc.
- Protogenic solvents: Solvents which have the tendency to produce protons, i.e., water, liquid hydrogen chloride, glacial acetic acid, etc.
- Amphiprotic solvents: Solvents which act both as protophilic or protogenic, e.g., water, ammonia, ethyl alcohol, etc.
- Aprotic solvents: Solvents which neither donate nor accept protons, e.g., benzene, carbon tetrachloride, carbon disulphide, etc.
HCI acts as acid in H3O+, stronger acid in NH3, weak acid in CH3COOH, neutral in C6H6and a weak base in HF.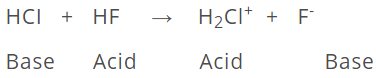
Lewis Concept of Acids and Bases
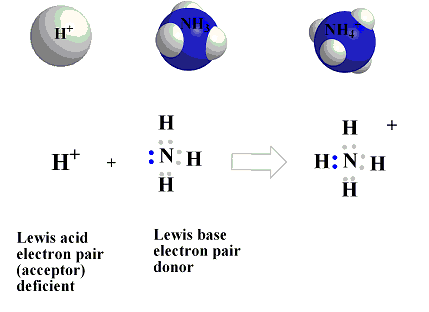
This concept was proposed by G.N. Lewis in 1939. According to Lewis's definition of acids and bases, a base is defined as a substance that can furnish a pair of electrons to form a coordinate bond, whereas an acid is a substance that can accept a pair of electrons. The acid is also known as an electron acceptor or electrophile, while the base is an electron donor or nucleophile. A simple example of an acid-base is the reaction of a proton with hydroxyl ion.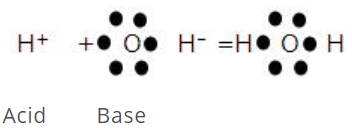
Some other examples are: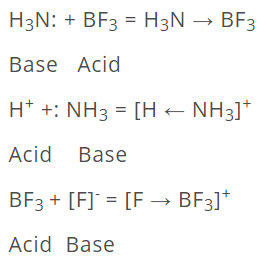
Lewis concept is more general than the Bronsted Lowry concept.
Lewis Acid & Lewis Base
According to Lewis concept, the following species can act as Lewis acids.
- Molecules in which the central atom has incomplete octet: All compounds having central atom with less than 8 electrons are Lewis acids, e.g., BF3, BC13, A1C13, MgCl2. BeCL. etc.
- Simple cations: All cations are expected to act as Lewis acids since they are deficient in electrons. However, cations such as Na+, K+, Ca2+, etc., have a very little tendency to accept electrons, while the cations like H+, Ag+, etc., have greater tendency to accept electrons and, therefore, act asLewis acids.
- Molecules in which the central atom has empty d-orbitals: The central atom of the halides such as SiX4, GeX4, TiCl4, SnX4, PX3, PF5, SF4, SeF4, TeCl4, etc., have vacant d-orbitals. These can, therefore, accept an electron pair and act as Lewis acids.
- Molecules having a multiple bond between atoms of dissimilar electronegativity: Typical examples of molecules falling in this class of Lewis acids are C02, S02 and S03. Under the influence of attacking Lewis base, one -electron pair will be shifted towards the more negative atom.
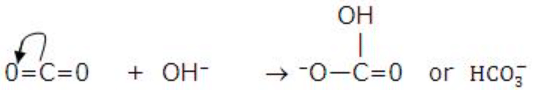
The following species can act as Lewis bases.
- Neutral species having at least one lone pair of electrons: For example, ammonia, amines, alcohols, etc., act as Lewis bases because they contain a pair of electrons
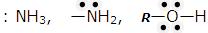
Negatively charged species or anions: For example, chloride, cyanide, hydroxide ions, etc., act as Lewis bases.CN-, CI-, OH-
- It may be noted that all Bronsted bases are also Lewis bases but all Bronsted acids are not Lewis acids.
Limitations of Lewis Concept of Acid and Base
- Since the strength of the Lewis acids and bases is found to depend on the type of reaction, it is not possible to arrange them in any order of their relative strength.
- The choice of which definition of acids and bases one wishes to use in a particular instance depends largely on the sort of chemistry that is studied.
- But Arrhenius concept is perfectly satisfactory and simplest for dealing with reactions in aqueous solutions.
- It explains satisfactorily the strength of acids and bases in aqueous solutions, neutralisation, salt hydrolysis, etc.
pH of Acids and Bases
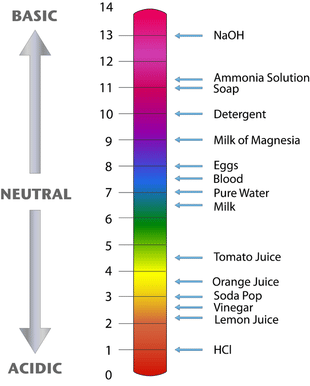
It is clear from the above discussion that nature of the solution (acidic, alkaline or neutral) can be represented in terms of either hydrogen ion concentration or hydroxyl ion concentration but it is convenient to express acidity or alkalinity of a solution by referring to the concentration of hydrogen ions only.
- pH measurement is done using pH paper and pH meter.
- Since H+ ion concentration can vary within a wide range from 1 mol per litre to about 1.0 × 10-14 mol per liter, a logarithmic notation was devised by Sorensen, in 1909, to simplify the expression of these quantities. The notation used is termed the pH scale.
- The hydrogen ion concentrations are expressed in terms of the numerical value of negative power to which 10 must be raised.
- This numerical value of negative power was termed as pH, i.e., pH = – log aH+ (Where aH+ is the activity of H+ ions).
- Activity of H+ ions is the concentration of free H+ ions in a solution.
- By free, we mean those that are at a large distance from the other ion so as not to experience its pull.
- We can infer from this that in dilute solutions, the activity of an ion is same as its concentration since more number of solvent molecules would separate the two ions.
- For concentrated solutions the activity would be much less than the concentration itself.
- Therefore, the earlier given expression of pH can be modified for dilute solutions as,
pH = – log [H+]. - This assumption can only be made when the solution is very much dilute, i.e, [H+] ≤ 1M.
- For higher concentration of H+ ions, one needs to calculate the activity experimentally and then calculate the pH.
- pH of a solution is, thus, defined as the negative logarithm of the concentration (in mol per litre) of hydrogen ions which it contains or pH of the solution is the logarithm of the reciprocal of H+ ion concentration.
- Just as pH indicates the hydrogen ion concentration, the pOH represents the hydroxyl ion concentration, i.e.,
pOH = -log [OH-] Considering the relationship,
[H+][OH-] = Kw = 1 x 10-14
Taking log on both sides, we have
log [H+] + log [OH-] = log Kw = log (1 x 10-14)
or -log [H+] - log [OH-] = -log Kw = -log (1 x 10-14)
or pH + pOH = PKw* = 14
i.e., sum of pH and pOH is equal to 14 in any aqueous solution at 25°C. The above discussion can be summarised in the following manner: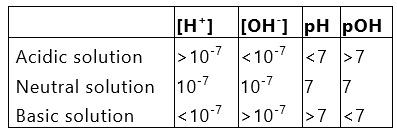
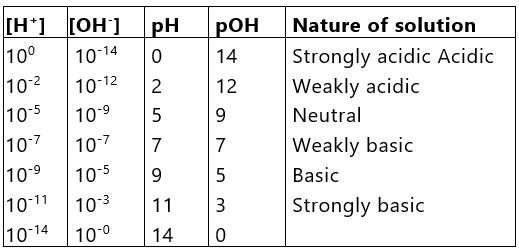
pH of Weak Acids and Bases
Weak acids and bases are not completely ionised; an equilibrium is found to have been established between ions and unionised molecule. Let us consider a weak acid of basicity 'n'.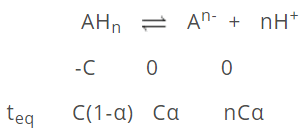
[H+] = nCα; .·. pH = -log10 [nCα]
For monobasic and, n=1
pH = -log10 [Cα]
Dissociation constant of acid Ka may be calculated as
Ka = [An-][H+]n/[AHn] = [Cα][nCα]n/[C(1-α)]
= α [nCα]n/(1-α) For weak acids, α« 1
.·. (1-α) = 1
= α[nCα ]n/(1-α)
nCKa = nCα [nCα ]n = [nCα ](n+1)
= [nCα ] = [nCKa]1/(n+1)
= [H+] = [nCKa]1/(n+1)
.·. pH = -1/(n+1) log10(nCKa)
For monobasic acid, n = 1
pH = -log√CKα
Since Ka = α[nCα]n
[nCα ] = [Kα/α]1/n = [H+]
pH = -1/n log10(Kα/α)
For n = 1 pH = -log10(Kα/α)
Limitations of pH Scale
- pH values of the solutions do not give us immediate idea of the relative strengths of the solutions. A solution of pH = 1 has a hydrogen ion concentration 100 times that of a solution of pH = 3 (not three times). A 4 x 10-5 N HCI is twice concentrated of a 2 x 10-5 N HCI solution, but the pH values of these solutions are 4.40 and 4.70 (not double).
- pH value of zero is obtained in 1 A' solution of strong acid. In case the concentration is 2 N, 3 N, 10 N, etc. The respective pH values will be negative.
- A solution of an acid having very low concentration, say 10-8 N, cannot have pH 8, as shown by pH formula, but the actual pH value will be less than 7.
Note:
Normality of strong acid = [H3O+]
Normality of strong base = [OH-]
.·. pH = -log [N] for strong acids
pOH = -log [N] for strong acidsSometimes pH of acid comes more than 7 and that of base comes less than 7. It shows that the solution is very dilute; in such cases, H+ or OH- contribution from water is also considered, e.g., in 10 N HCI,
[H+]Total = [10-8]Acid + [10-7]Water
= 11 × 10-8 M= 1.1 × 10-7 M
pH of Mixture
Let one litre of an acidic solution of pH 2 be mixed with two litre of other acidic solution of pH 3. The resultant pH of the mixture can be evaluated in the following way.
MlVl+M2V2 = MR(Vi + V2) 10-2 × 1 + 10-3 × 2 = MR (1 +2)
(12 + 10-3)/3 = MR
4 × 10-3 = MR(Here, MR = Resultant molarity)
pH = -log (4 × 10-3)
Total concentration of [H+] or [H3+O ] in a mixture of weak acid and a strong acid = (C2 + √(c22 + 4KaC1 ))/2
where C1 is the concentration of weak acid (in mol litre having dissociation constantKa
C2 is the concentration of strong acid
Total [OH-] concentration in a mixture of two weak bases = √(K1C1+ K2C2)
where K1 and K2 are dissociation constants of two weak bases having C1 and C2 as their mol litre-1 concentration respectively.
Periodic Variations of Acidic and Basic Properties
(a) Hydracids of the Elements of the Same Periods
Consider the hydracids of the elements of II period, Viz., CH4, NH3, H20 and HF. These hydrides become increasingly acidic as we move from CH4 to HF. CH4 has negligible acidic properties while HF is a fairly stronger acid. The increase in acidic properties is due to the fact that the stability of their conjugate bases increases in the order
CH-3< NH-2 < OH- < F-
The increase in acidic properties is supported by the successive increase in the dissociation constant.
CH4(=10-58)<NH3 (=10-35)<H20(=10-14)<HF(=10-4)
(b) Hydracids of the Elements of Same Group
- Hydrides of V group elements (NH3, PH3, AsH3, SbH3, BiH3) show basic character which decreases due to increase in size and decrease in electronegativity from N to Bi. There is a decrease in electron density in, sp3 -hybrid orbital and thus electron donor capacity decreases.
- Hydracids of VI group elements (H20, H2S, H2Se, H2Te) act as weak acids. The strength increases in the order H20 < H2S < H2Se < H2Te. The increasing acidic properties reflects decreasing trend in the electron donor capacity of OH-, HS-, HSe- or HTe- ions.
- Hydracids of VII group elements (HF, HCI, HBr, HI) show acidic properties which increase from HF to HI. This is explained by the fact that bond energies decrease. (H-F = 135 kcal/mol, HCI = 103, HBr = 88 and HI = 71 kcal/mol).
(c) Oxyacids
The acidic properties of oxyacids of the same element which is in different oxidation states increases with increase in oxidation number.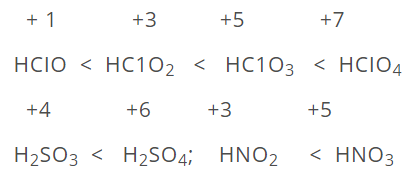
But this rule fails in oxyacids of phosphorus.
H3PO2 > H3PO3 > H3PO4
The acidic properties of the oxyacids of different elements which are in the same oxidation state decreases as the atomic number increases. This is due to increase in size and decrease in electronegativity.
HC1O4 > HBrO4 > HIO4
H2SO3 > H2SeO3
But there are a number of acid-base reactions in which no proton transfer takes place, e.g.,
SO2 + SO2 ↔ SO2+ + S
Acid1 Base2 Acid2 Base1
Thus, the protonic definition cannot be used to explain the reactions occurring in non-protonic solvents such as COCl2, S02, N2O4, etc.
Example: The hydrogen ion concentration of a solution is 0.001 M. What will be the hydroxyl ion concentration of solution?
Solution: We know that [H+][OH-] 1.0 × 10-14
Given that,
[H+] = 0.001 M = 10-3 M
So, [OH-] = 1.0 * 10-14/[H+] = (1* 10-14)/10-3 = 10-11M
|
110 videos|130 docs|117 tests
|
FAQs on Acids and Bases - Chemistry for ACT
| 1. What is the Arrhenius concept of acids and bases? |  |
| 2. What is the Bronsted-Lowry concept of acids and bases? |  |
| 3. How does the Lewis concept define acids and bases? |  |
| 4. What is the difference between a Lewis acid and a Lewis base? |  |
| 5. How are the Lewis concept and the Bronsted-Lowry concept related? |  |





















