Air and Water (Part 2) Class 5 Notes Science
Water
Water is essential for the survival of all living things. Plants, animals and human beings need water. We use water for drinking, cooking, washing, bathing, cleaning, etc. The earth’s surface is made up of almost 71% water. About 97% of this water is present in the oceans and seas. This water is salty. 2% of water is present in the form of ice and in glaciers. Only 1% of water is available to us as fresh water in rivers, lakes and ponds.

Properties of water
- Pure water is colourless, tasteless and odourless.
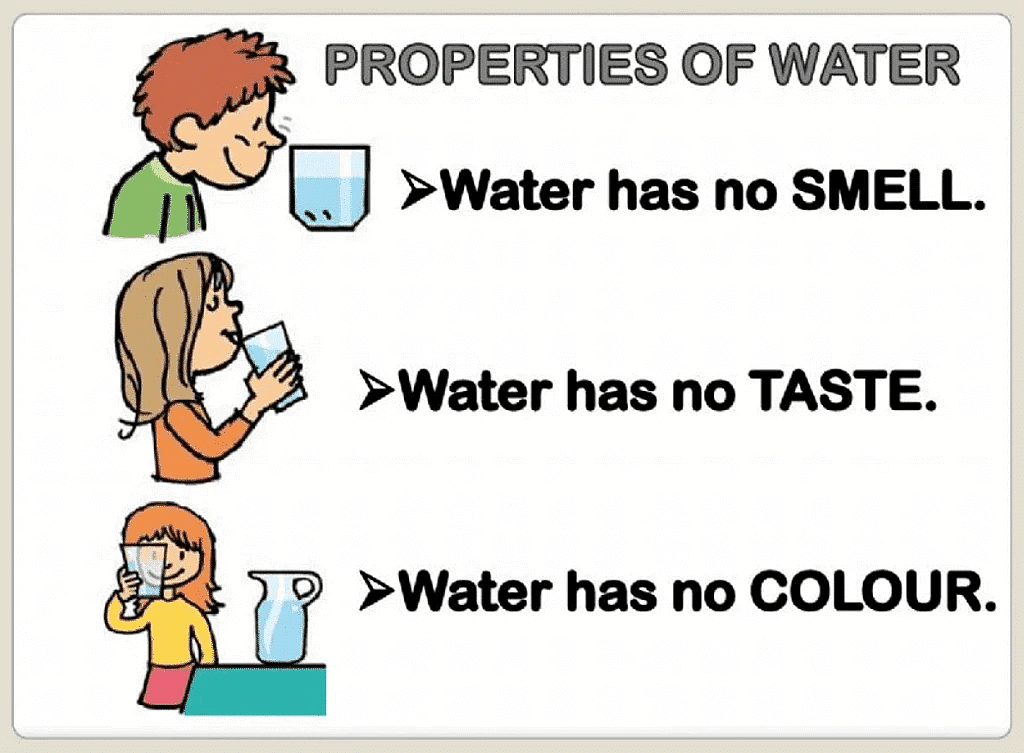
- Water is called the universal solvent because it dissolves many things.
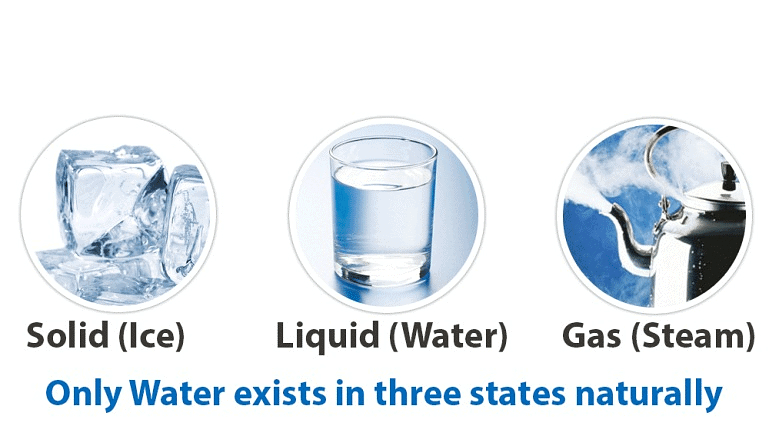
- It is the only natural substance that is found in all three states of matter—solid, liquid and gas.
- It is valuable to industries because it absorbs a lot of heat.
- Water tends to clump together in drops. Due to this reason, it can move through the roots and stem of plants and through tiny blood vessels in our body.
Impurities in Water
The substances that make water dirty or unfit for use are called impurities. The impurities are of two kinds – soluble and insoluble impurities. Soluble impurities are salts and minerals that dissolve in water. Insoluble impurities are pieces of stone, sand, mud, chalk and several other substances. Sometimes water also contains germs which cause diseases.
Removing Insoluble Impurities
Insoluble impurities can be removed by sedimentation, decantation and filtration.
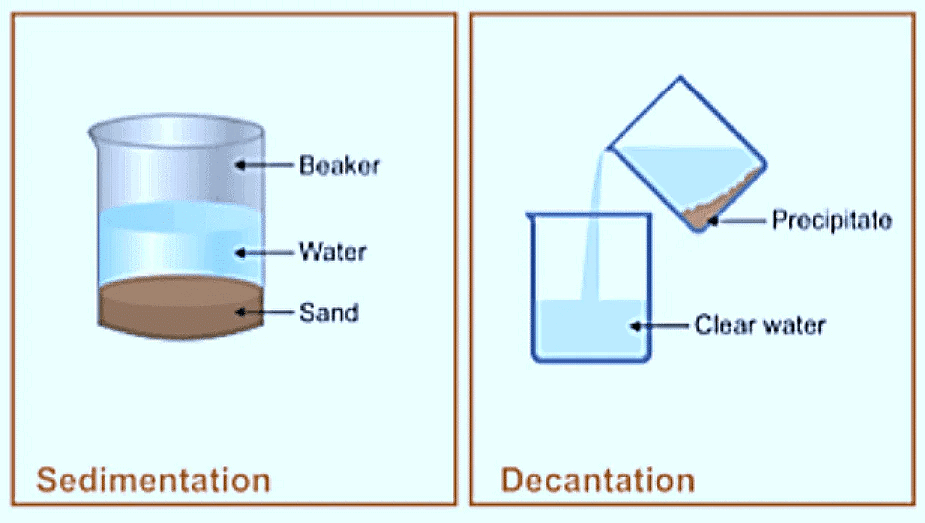
1. Sedimentation and decantion:
- Water with insoluble impurities are allowed to stand, undisturbed. After some time, the insoluble impurities settle down at the bottom of the container as sediments. This process is called sedimentation.
- After sedimentation, the clean water can be slowly poured out into a separate container. This process is called decantation.
- The rate of sedimentation can be improved by adding some chemicals like alum. This process is called loading. Alum particles attach themselves to the impurities, thus, making them heavier.
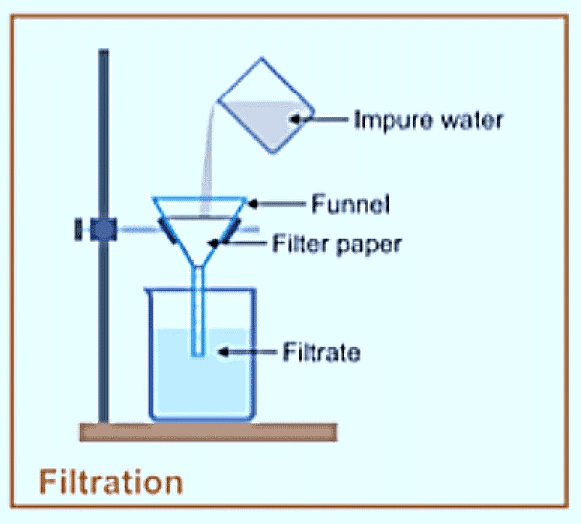
2. Filteration:
- In this process liquid is passed through a filter paper.
- A mixture of sand and water can be filtered.
- The filter paper is folded twice to make a cone. This cone is kept inside a funnel.
- The funnel is kept on the conical flask and impure water containing sand is poured into the funnel.
- The water seeps through the filter paper and collects in the flask.
- Impurities like sand are left on the filter paper.
Removing Soluble Impurities
It is more difficult and expensive to remove soluble impurities from water. Soluble impurities can be removed by evaporation or distillation.
1. Evaporation:
- Evaporation is the simplest way of separating any dissolved solid in a liquid.
- In this process, the impure water is heated to boil.
- Upon heating, the liquid water turns into water vapours, and the dissolved impurities are left behind in the vessel.
- This method is also used on large scale to obtain salt from sea water in hot countries.
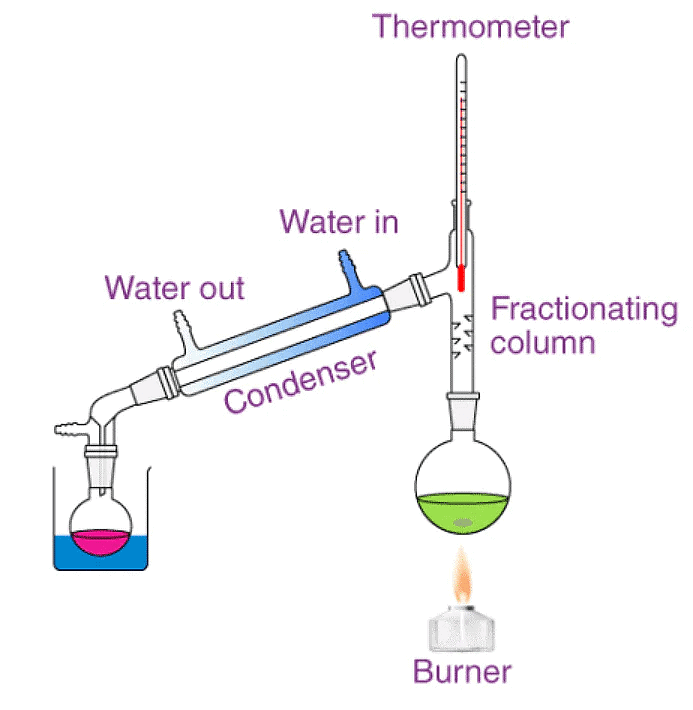
2. Distillation:
- In evaporation, the water is lost in the form of water vapour.
- To get pure water we have to cool the water vapour so that it condenses to form water again.
- This is done by heating the water in a flask.
- The water vapour passes through a tube into another flask.
- This flask is kept cool by cold water. Here the water vapour condenses.
- The water collected in this way is called distilled water.
Purifying Drinking Water
Contaminated water may contain germs and can spread diseases. It is essential to drink clean water.
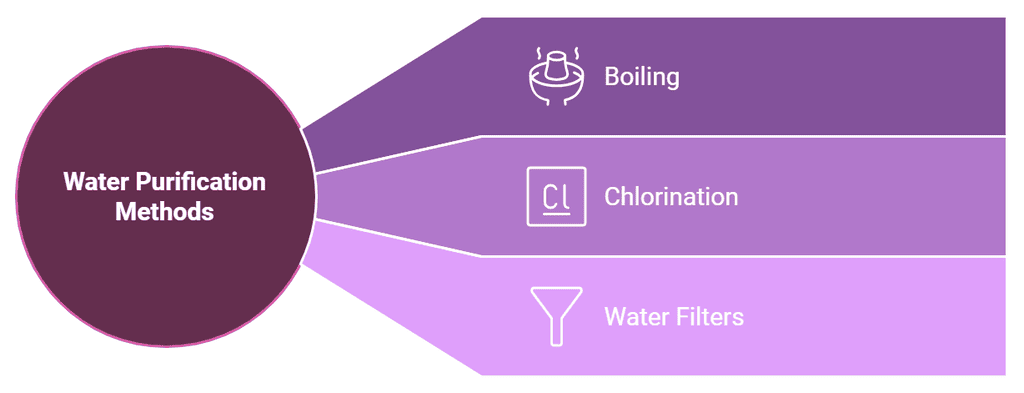 Water Purification Methods
Water Purification Methods
- Boiling: It is the simplest way to purify drinking water. Boiling water for about 10 minutes kills the germs present in it.
- Chlorination: The process of adding chlorine to water for purifying it is called chlorination. Chlorine tablets are easily available in the market. Chlorine kills the germs in water and makes it safe for drinking.
- Water Filters: Water filters can also be used to purify water at home.
|
42 videos|380 docs|45 tests
|
FAQs on Air and Water (Part 2) Class 5 Notes Science
| 1. What are the common impurities found in water? |  |
| 2. How do impurities affect the quality of water? |  |
| 3. How can we remove impurities from water? |  |
| 4. What are the health risks associated with drinking water containing impurities? |  |
| 5. How can we ensure the purity of drinking water at home? |  |

















