Group-2 Elements: Alkaline Earth Metals | Chemistry for JEE Main & Advanced PDF Download
What are Alkaline Earth Metals?
Elements whose atoms have their s-subshell filled with their two valence electrons are called alkaline earth metals. Their general electronic configuration is [Noble gas] ns2. They occupy the second column of the periodic table and so-called as group two metals also.
Examples of Alkaline earth Metals: Beryllium (Be), Magnesium(Mg), Calcium (Ca), Strontium (Sr), Barium(Ba) and Radium (Ra).
They occupy successive periods from first to seven of this radium is a radioactive element. Alkaline earth metals form amalgams with mercury.
Overview of Alkaline Earth Metals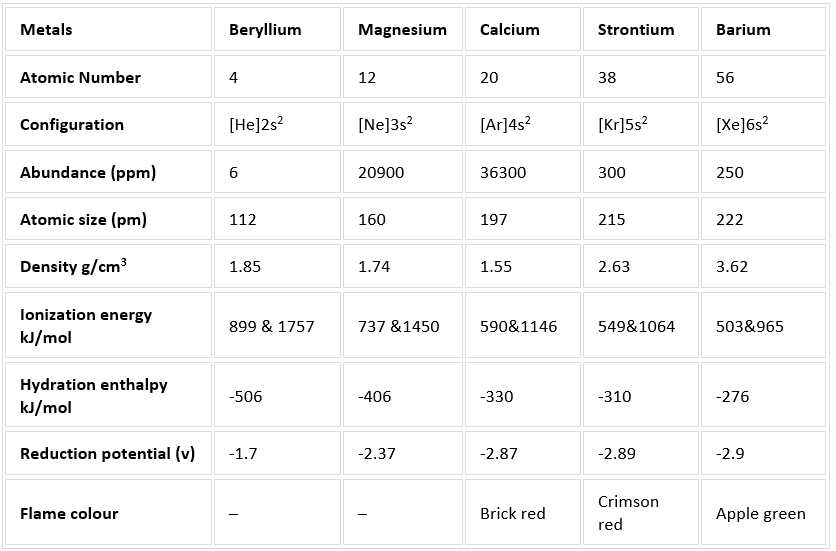
The alkaline earth metals are the elements that correspond to group 2 of the modern periodic table. This group of elements includes beryllium, magnesium, calcium, strontium, barium, and radium. The elements of this group are quite similar in their physical and chemical properties. For example, all alkaline earth metals are silvery-white coloured solids under standard conditions. They are also highly lustrous (shiny) and are quite reactive. The general electronic configuration of these elements is ns2. Since the alkaline earth metals have a completely full s-orbital in their respective valence shells, they tend to readily lose two electrons to form cations with a charge of +2. Thus, the most common oxidation state exhibited by the alkaline earth metals is +2.
Physical Properties of Alkaline Earth Metals
Down the column, nuclear charge increases and a new orbital is added to each alkaline earth atom.
Atomic and Ionic Radii
Ionic and Atomic radius increases down the column of the periodic table, both radii will be smaller than the alkali metal and larger than other atoms of the same period due to charge and addition of the electron to the same energy level.
Alkaline earth elements can lose both s-electrons and hence become doubly positive cationic. The cationic radius is smaller than the neutral atom. Still, the ionic radii increase down the column. For Example, RBe ˂ RMg ˂ RCa ˂ RSr ˂ RBa and RBe2+ ˂ RMg2+ ˂ RCa2+ ˂ RSr2+ ˂ RBa2+
Why Alkaline Earth Metals are Denser than Alkali Metals?
Radii being smaller, the volume of the atoms are also smaller. In addition, due to the presence of two valence electrons, atoms have stronger metallic bonding. Hence, alkaline earth metals have more density and harder than alkali metals.
Density generally increases from magnesium to radium while calcium has the lowest density among the alkaline earth metals.
Ionization Energy
Alkaline earth elements can donate both valence electrons to get a noble gas configuration of octet configuration. Thus, they have two ionization energies:
1. First Ionization energy
The first ionization energy of alkaline earth metals is the energy needed for the removal of the first electron from the neutral atom. It is larger than that of the alkali metal atom for two reasons:
- Due to smaller radii and the electrons being held tightly by the higher nuclear charge, and
- Electron being removed from a fully filled and hence a stable subshell.
2. Second Ionization Energy
The second ionization energy of alkaline earth metals needed for the second electron from the cation will be more than the first ionization energy of the atom, but less than any second ionization of alkali metal. In spite of the high ionization energy, removal of both electrons are feasible because,
- Atom gets a noble gas configuration
- The smaller size and higher charge help to overcome the higher ionization energy by higher lattice energy arising due to the close packing of atoms or ions in solids.
- Higher hydration energy in liquids due to larger solvation.
So group two alkaline earth elements are all divalent electropositive metals and exhibit a fixed oxidation state of 2. Ionization energy needed for the removal of the valence electron will be highest for the small beryllium atom.
With increasing atomic size, the valence electron gets shielded by the inner electrons and becomes easily removable with less energy requirement. Hence the ionization energy decreases with an increasing atomic number or atomic size.
Example: IEBe > IEMg > IECa > IESr > IEBa
Note: In the same period ionization energy increases due to decreasing the ionic size and increasing nuclear charge.
Why Solubility of Alkaline Earth Metals decreases down the Group?
Beryllium ion is the most soluble and the solubility decreases with increasing size so that Barium ion is the least water-soluble alkaline earth metal ion. Solubility in water is related to the ionic nature and size.
Smaller ions have higher charge density and can be solvated by more water molecules. This releases a higher enthalpy of hydration and makes the hydrated ions more stable.
Example: Solubility of Be2+ > Solubility of Mg2+ > Solubility of Ca2+ > Solubility of Sr2+ > Solubility of Ba2+
Reactivity of Alkaline Earth Metals
Reducing ability is inversely related to ionization energy. As ionization energy decreases down the column, reducing property is expected to increase from Beryllium to Barium.
Reduction potential also decreases from beryllium to barium indicating the increasing reducing capacities. But, the alkaline earth metals are weaker reducing agents than alkali metals, due to higher ionization energy.
Flame Colouration
In Alkaline Earth Metals, the energy needed for an electronic transition between the available energy levels falls in the visible spectrum region. So, on heating, except beryllium and magnesium produce a characteristic colour to the flame reflective of their emission or absorption spectrum and can be used for their identification.
Example: Ca – Brick Red colour, Sr – Crimson Red colour, and Ba – Apple Green colour.
Melting and Boiling Points
Because of smaller size and strong metallic bonding in close-packed structure, the melting and boiling points of the alkaline earth metals are higher than alkali metals. Among the alkaline earth metals except for magnesium, the melting and boiling points decrease regularly from beryllium to barium.
Chemical Properties of Alkaline Earth Metals
The key features of the compounds of alkaline earth metals and their general characteristics are discussed in this subsection.
1. Hydrides
Beryllium does not react with hydrogen directly. Beryllium hydride can be prepared by the reduction of beryllium chloride with lithium aluminium hydride.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Beryllium and magnesium form covalent hydrides where each hydrogen is connected to two metal atoms. This is an example of molecules with three centres sharing only two electrons called “banana Bond”.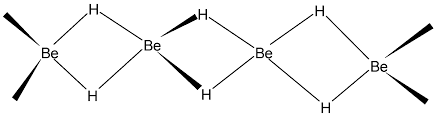 Hydrides of Alkaline Earth Metals
Hydrides of Alkaline Earth Metals
Calcium, strontium and barium react with hydrogen to form metallic hydrides. Metallic hydrides give hydrides ions.
M + H2 → 2MH2 → M+ + 2 H–
Hydrides react violently with water to release hydrogen. Calcium hydride called “Hydrolith” is used for producing hydrogen.
CaH2 + 2H2O → Ca(OH)2 + H2
2. Reaction of Alkaline Earth Metals with Water
Beryllium does not react with water even at higher temperatures. Magnesium reacts with hot water only to form hydroxides and releasing hydrogen. Magnesium gets a protecting coat of its oxide, that prevents any further attack by the water molecules. Other alkaline earth metals react with even cold water to liberate hydrogen.
3. Carbides
Alkaline earth metals and their oxides, except beryllium, react with carbon to yield carbides. Carbides react with water to liberate acetylene gas and hence used as a source for the gas.
M + 2C → MC2 MC2 + 2H2O → M(OH) 2 + C2H2
4. Oxides
Beryllium reacts with oxygen only above 600°C. Magnesium and strontium burn in oxygen to form oxides while Barium forms peroxides.
BeO and MgO are more covalent while the other oxides are ionic. Beryllium oxide is amphoteric, magnesium oxide and calcium oxide are weakly basic while other oxides are basic.
5. Hydroxides
Oxides react with water to ultimately yield hydroxides. The basic nature and the thermal stability of hydroxides increases from beryllium to barium.
6. Carbonates and Bicarbonates
The hydroxides react with carbon dioxide to carbonates.
M(OH)2 + CO2 → MCO3 + H2O
Bicarbonates are soluble in water and exist only in solution. Carbonates exist as solid and insoluble in water. The solubility of carbonates decreases from Be to Ba. In the presence of carbon dioxide, carbonates dissolve by forming bicarbonates. Ionic character and the thermal stability of the carbonates increases from Be to Ba.
7. Sulphates
Contrary to alkali metal sulphates, beryllium sulphate is water-soluble. The smaller size and the charge density increases the hydration energy of the beryllium sulphate leading to more solubility. In other sulphates, increasing lattice energy and the decreasing hydration energy (due to increasing size) decreases their solubility form BeSO4 to BaSO4.
Solubility of BeSO4 > MgSO4 > CaSO4 > SrSO4 > BaSO4
8. Nitrates
Nitrates can be prepared by reacting the corresponding oxides, hydroxides and carbonates with nitric acid. Nitrates are soluble in water. On heating, Beryllium nitrate forms nitrite and, other nitrates yield oxide, liberating brown fumes of nitrogen dioxide.
2M(NO3)2 → 2MO + 4NO2 + O2
9. Halides
Alkaline earth metals from calcium to barium react with all halogens to form solid ionic halides with a definite crystal structure. Reactivity decreases from fluorine to iodine. Beryllium halides are an exception with more covalent bonding because of the high polarization of the small covalent ion on the electron cloud of the halogen anion as indicated by the Fajan’s rule.
In the gas phase, Beryllium halides exist as individual molecules and in the solid phase, they form chains of Be-X.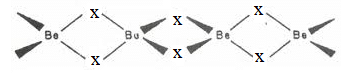 Halides of Alkaline Earth Metals
Halides of Alkaline Earth Metals
Fluorides are insoluble in water. The solubility of other halides decreases with increase in ionic size i.e. from Mg2+ to Ba2+. Halides are hygroscopic and have the water of crystallization in their solid state (CaCl2.6H2O). Fused halides are used as dehydrating agents.
Reaction of Alkaline Earth Metals with Liquid Ammonia
Like alkali metals, Alkaline earth metals also form ammonia solvated cation and electrons. The solution is electrically conductive, reductive and paramagnetic. The solvated electrons absorb in the visible region and the solution turns blue in colour. The concentrated solution is bronze in colour. On long standing, it decomposes into amide, ammonia and hydrogen.
M + (x + y) NH3 → M(NH3)x]+ + [M(NH3)y]– → MNH2 + 1/2H2
Complex of Alkaline Earth Metal
Smaller alkaline earth metals form complexes. Beryllium forms many complexes with mono, di and tetradentate ligands.
Examples: [BeF3]– , [BeF4]2-, [Be(H2C2O4)]2-, [Be4O(R)6], where R may be NO3–, HCOO–, CH3COO– etc.
Anomalous behaviour of Beryllium
Beryllium has more covalent nature due to its smallest size, Highest ionization energy, high electropositive nature and strongest polarizing nature. Because of these, Beryllium differs from other alkaline earth metal properties.
- It is the hardest metal among alkaline earth metals
- Does not react with water even at red hot conditions.
- Melting and boiling point of beryllium is maximum.
- It does not react directly with hydrogen to form hydride.
- Unlike other alkaline earth metals, does not liberate hydrogen from acid because of higher electrode potential.
- Concentrate nitric acid form a coating of oxide, which makes it passive.
- Beryllium oxide and hydroxide are amphoteric. Dissolves in acids to form salts and in bases to form beryllate.
- Beryllium forms carbide of a different formula and yields methane and not acetylene like other metal on reaction with water.
- Beryllium nitride is volatile.
- It does not react with atmospheric nitrogen and oxygen.
Diagonal Relationship of Beryllium with Aluminium
Beryllium of group two resembles more with Aluminum of group three:
- Both beryllium and aluminium occur together in the mineral, “Beryl” 3BeO Al2O3 6SiO2
- Both of them do not react with atmospheric oxygen and nitrogen.
- Both of them do not react with water even at high temperatures.
- They do not liberate hydrogen from acid.
- On treatment with concentrated nitric acid, they become passive.
- Both form polyvalent bridged hydrides of covalent nature.
- Halides of both are polyvalent, bridged, and of low melting points. Halides are Lewis acids.
- Water hydrolyzes both nitrides liberate ammonia. Oxides and hydroxides of Be and Al are amphoteric. So, they react with acid as well base.
- Both form carbide, that on hydrolysis yields Methane.
- Carbonates of beryllium and Aluminum are unstable.
Uses of Alkaline Earth Metals
1. Calcium Carbonate
It occurs naturally in many forms as marble, limestone, chalk, coral calcite etc. The pure form is made by:
- First, dissolve the mineral in hydrochloric acid,
- Removing, hydroxide forming impurities like iron, aluminium, by the addition ammonia,
- Finally, precipitating the calcium carbonate by the addition of ammonium carbonate.
Limestone on heating decomposes to evolve carbon dioxide and form quick lime (CaO).
CaCO3 → CaO + CO2
Calcium oxide (quick lime) reacts exothermically with water to form calcium hydroxide (lime water or slaked lime).
CaO + H2O → Ca(OH)2
2. Plaster of Paris [Calcium hemihydrate, CaSO4.1/2H2O]
Naturally, available gypsum is calcium sulphate dihydrate (CaSO4. 2 H2O). It exists in the monoclinic crystal structure. An aqueous solution of soluble calcium salts like nitrates or chlorides on treatment with dilute sulphuric acid precipitates out hydrous calcium sulphate.
On heating in a carbon-free environment (otherwise calcium sulphate is reduced to calcium sulphite), depending on the temperature monoclinic gypsum undergoes various transformations. It hardens first into another orthorhombic allotropy form.
- At 120°C: Some of the water of hydration is last to yield calcium sulphate hemihydrate, called as Plaster of Paris.
- On heating to 200°C: It loses the remaining water and becomes anhydrous calcium sulphate called “dead burnt Plaster”.
- At 400°C: Calcium Sulphate decomposes into calcium oxide and evolving Sulphur dioxide and oxygen.
Properties
- A paste of this hemihydrate with about one-third of water sets to a hard mass, in any moulding, in about 15 minutes. Added water may rehydrate the hemihydrate into dihydrate.
- Salts like sodium chloride accelerates the hydration to reduce the setting time, while alum or borax reduce the hydration to increase the setting time of hardening.
- It is used much in decorating surfaces, making false ceilings, bondages in surgical treatment, dentistry, etc.
|
352 videos|596 docs|309 tests
|
FAQs on Group-2 Elements: Alkaline Earth Metals - Chemistry for JEE Main & Advanced
| 1. What are alkaline earth metals? |  |
| 2. What are the main characteristics of alkaline earth metals? |  |
| 3. How do alkaline earth metals react with water? |  |
| 4. What are the common uses of alkaline earth metals? |  |
| 5. Are alkaline earth metals toxic? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|


















