Alkaloids-Bio-Molecules | Organic Chemistry PDF Download
What is Alkaloids?
Alkaloids means ‘Alkali’ like. Alkaloids are defined as natural plant compounds that have a basic character and contain at least one nitrogen atom in a heterocyclic ring and have biological activities. The Pharmacist W.Meissner proposed the term Alkaloids in 1819. Alkaloids (alkali = base, oid=like sub). Characteristic features: Alkaloids are basic nitrogenous plant origin, mostly optically active & possessing nitrogen hetero cycles as their structural units with physiological action. The previous definitions not fully correct because they do not follow on all alkaloids for e.g.
- Colchicine: Colchicine is regarded as an alkaloid although it is not heterocyclic and is scarcely basic.
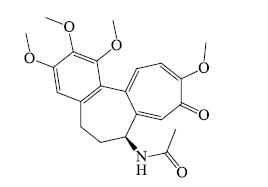
- Thiamine: It is heterocyclic nitrogenous base but not as a alkaloid because it is universally distributed in living matter.
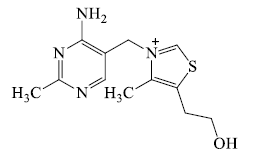
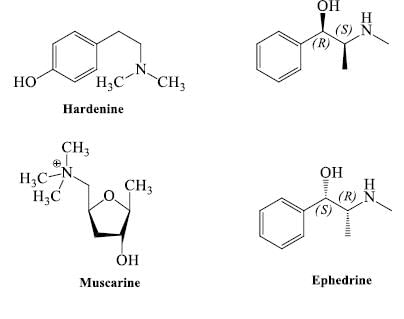
- Nitrogen as side chain: Some compound is classed as in alkaloids but they do not contain nitrogen in heterocyclic ring, but contain nitrogen inside the chain e.g. ephedrine, hordenine, betanine, muscarine, strychnine & tryptamine etc.
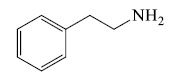
- Naturally occurring open chain basic compound: These compounds have physiological activity but do not class in alkaloids e.g. Cholines, amino acid, phenylethylamines etc.
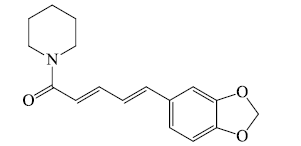
- Piperine: It is neither basic character nor possessing any physiological activity but include in alkaloids.
Those compound, which fully satisfy the definitions, like physiological active, heterrocyclic basic nitrogenous ring but they do not classed in alkaloids e.g.- Thiamine, caffeine, purine, theobromine, and xanthenes.
Occurrence of Alkaloids
Alkaloids are chemically nitrogenous heterocyclic basic compound occur in nature, about 15% of vascular plant & widely distributed in higher plant e.g.. -Apocynace, papaveraceae, papilanaceae, rananeulaceae, solenaceae.They are present in the form of salts of organic acid, like acetic acid, oxalic acid, malic, lactic, tartaric, tannic, aconitic acid, few are with sugar e.g. Solanum, veratrum groups. Acc. to parts of plants:
- Leaves: Nicotine
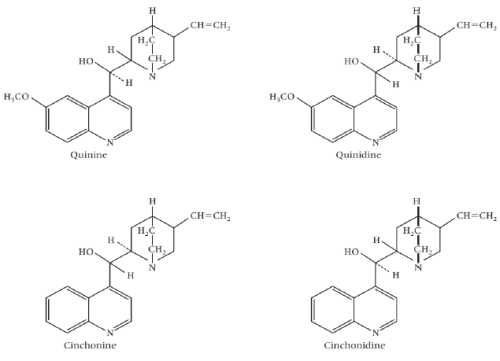
- Bark: Cinchonine, Quinine.
- Seeds: Strychnine, Nibidine.
- Roots: Rawelfinine, Glycyrrhizin, Punarnavine I & II
NOMENCLATURE
There was no systematic nomenclature. But there are some methods for nomenclature which are mention below.
1. According to their source: They are named according to the family in which they are found e.g. papavarine, punarnavin, ephedrin.
2. According to their Physiological response: They are named according to their physiological response e.g.. Morphine means God of dreams, Emetine means to vomit.
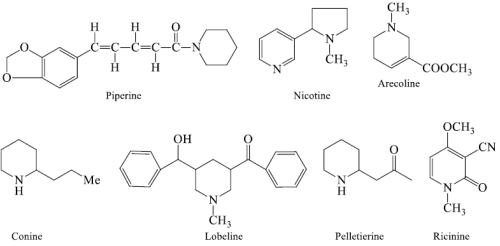
3. According to their discovery: They are named according to their discovery e.g. Pelletierine has been named on its discoverer, P.J. Pelletier.
4. Prefixes: They are named by some prefixes which are fix in nomenclature of alkaloids, e.g. epi, iso, neo, pseudo etc.
CLASSIFICATION
Based on the chemical nature
Alkaloids are classified into two major groups as mentioned below:
1. Heterocyclic or typical alkaloids
2. Non-heterocyclic or atypical alkaloids.
1. Heterocyclic Alkaloids
i). Pyridines and piperidines based
ii) Quinolines based
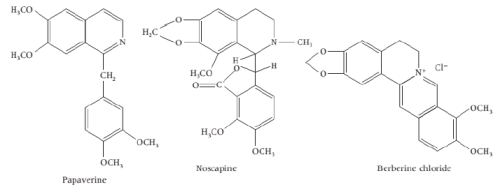
iii) Isoquinolines based
iv) Phenanthrenes based
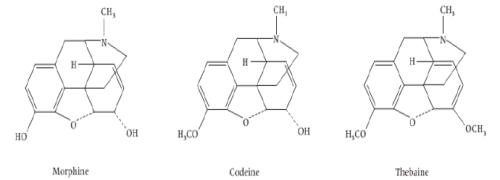
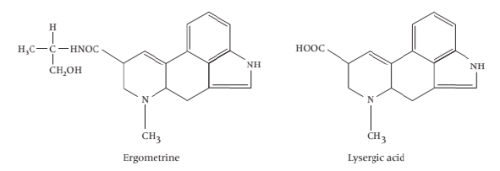
v) Indole based
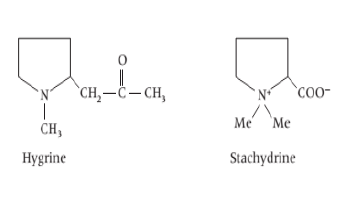
vi) Tropane based
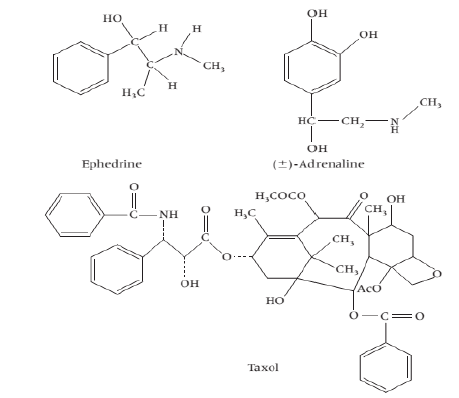
Non-heterocyclic Alkaloids
Degradation of Alkaloids
Degradation of Alkaloids: For discovering the structural system which incorporate these substituents groups & is tackled by degradation of the molecules by following methods:
1. Hoffmann's exhaustive methylation: - This is a composite reaction of alkaloid (Heterocyclic amines) and involves following steps:
(a) The alkaloid is treated with excess of CH3I to form quartertionarey -ammoniumiodide.
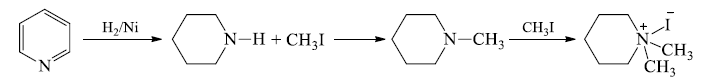
(b) 40-ammonium iodide is converted to the hydroxide and heated. The -OH of hydroxide extracts hydrogen atom from beta position and eliminate a water molecule and also the ring is cleaved at the N-atom to give an open chain 30-amine.
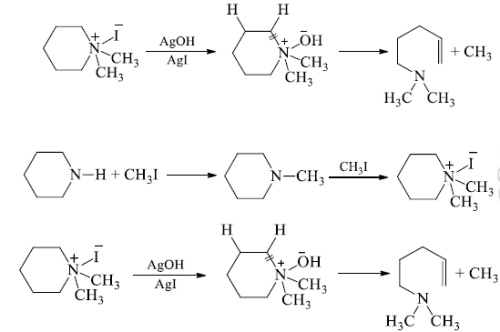
(c) The step Ist and IInd are repeated when a second cleavage at the N-atom given an unsaturated hydrocarbon which isomerase’s to conjugated derivative.
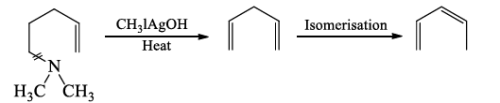
The exhaustive methylation of an alkaloid is an important method for the investigation of the nature of the C-skeleton in the heterocyclic system.
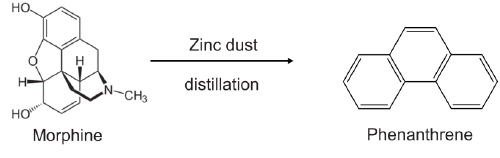
2. Zinc distillation: Distillation of alkaloid over zinc dust degrades it into a stable aromatic derivative.
Structure Elucidation of Caffeine
- Caffeine when subjected to Herzig Meyer’s method of N-methyl determination gives 3 moles of methyl iodide and xanthine, indicating the presence of 3-N-methyl groups.
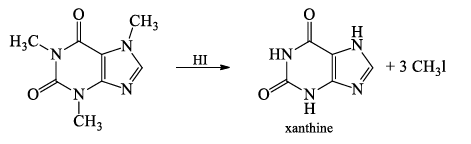
- Caffeine when oxidized with potassium chlorate in hydrochloric acid solution, yielded equimolar amounts of 1,3-dimethylalloxan, monomethyl urea, and N-methylhydantoin.

- 1,3-Dimethyl alloxan on further hydrolysis gave N, N’-dimethyl urea and mesoxalic acid.
- N-methylhydantoin on hydrolysis afforded N-methyl glycine together with CO2 and ammonia.
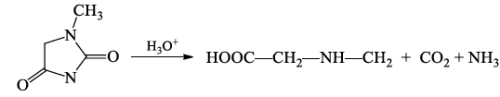
- The formation of 1,3-dimethyl-alloxan indicated the presence of pyrimidine ring containing two methyl groups. Similarly formation of N-methylhydantoin revealed the presence of imidazole ring with one N-methyl substituent.
- Thus oxidation studies established the position of two methyl groups at 1 and 3 positions of xanthine skeleton of caffeine.
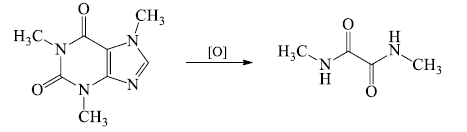
- The position of third methyl group, which may be at 7 or 9, was fixed from further oxidative degradation.
- Caffeine on chlorination gave chloro-caffeine, which on nucleophilic displacement with methoxide ion yielded methoxycaffine the hydrolysis of the latter compound yields oxycaffeine.
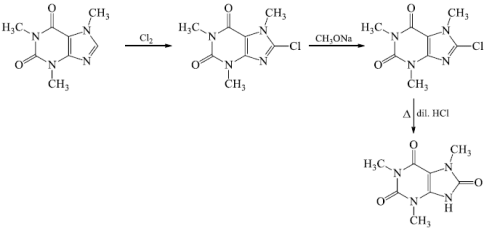
The structure of caffeine was further confirmed by total synthesis. Traube synthesized caffeine starting from N, N’-dimethyllurea and ethylcyanoacetate.
Physicochemical properties:
- Solid crystalline compound (exception are: coniine and Nicotine are liquid (It doesn't have Oxygen in their structure).
- Colorless compound (exception are berberine (yellow), Betaine (red).
- Sharp melting Point because it’s pure compound in crystal form.
- Can be either 1º, 2º, 3º or 40 alkaloid
- Basicity depends on availability of lone pair of electrons:
1. Electron donating or electron withdrsawing neighbors.
2. Type of hybridization.
3. Aromaticity.
|
44 videos|102 docs|52 tests
|
FAQs on Alkaloids-Bio-Molecules - Organic Chemistry
| 1. What are alkaloids? |  |
| 2. How do alkaloids act as bio-molecules? |  |
| 3. Which plants are rich sources of alkaloids? |  |
| 4. What are some medicinal applications of alkaloids? |  |
| 5. Can alkaloids have harmful effects on the body? |  |
















