Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Amounts of Substances in Equations
Amounts of Substances in Equations | Chemistry for Grade 10 PDF Download
Calculating Masses from Balanced Equations
Higher Tier Only
- Chemical equations can be used to calculate the moles or masses of reactants and products
- To do this, information given in the question is used to find the amount in moles of the substances being considered
- Then, the ratio between the substances is identified using the balanced chemical equation
- Once the moles have been determined they can then be converted into grams using the relative atomic or relative formula masses
Solved Example
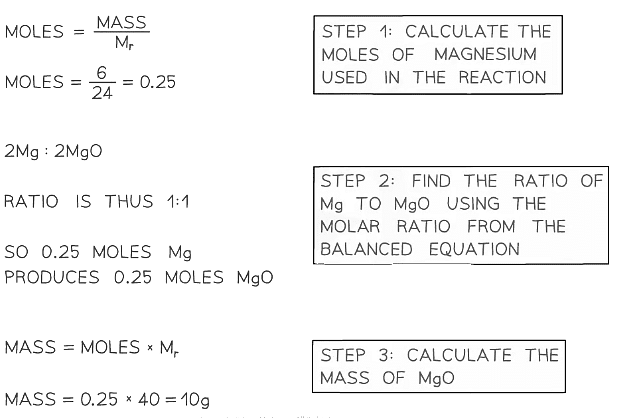
Example 1: Calculate the mass of magnesium oxide that can be made by completely burning 6.0 g of magnesium in oxygen in the following reaction: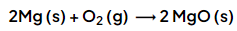
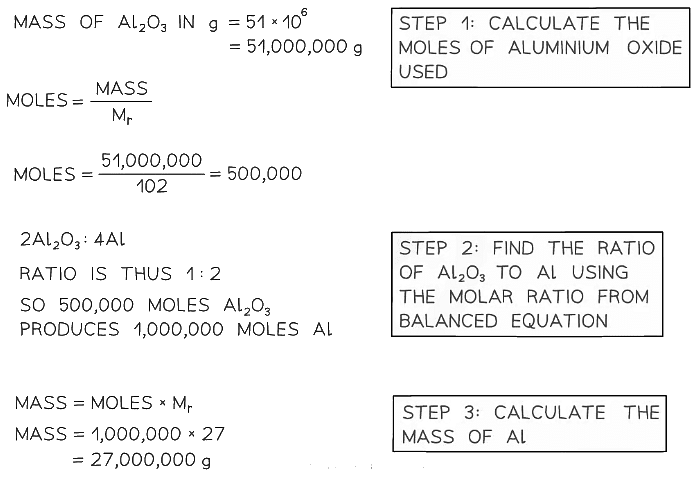
Example 2: Calculate the mass of aluminium, in tonnes, that can be produced from 51 tonnes of aluminium oxide. The equation for the reaction is: 2Al2O3 ⟶ 4Al + 3O2
Balancing Equations using Reacting Masses
- If the masses of reactants and products of a reaction are known then we can use them to write a balanced equation for that reaction
- This is done by converting the masses to moles and simplifying to find the molar ratios
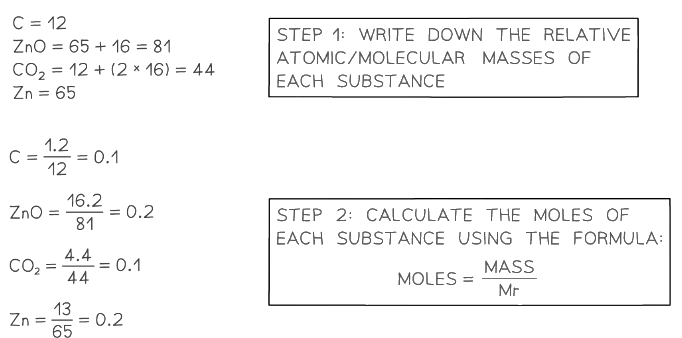
Example 3: A student reacts 1.2 g of carbon with 16.2 g of zinc oxide. The resulting products are 4.4 g of carbon dioxide and 13 g of zinc. Determine the balanced equation for the reaction.
Exam Tip
- These questions look hard but they are actually quite easy to do, as long as you follow the steps and organise your work neatly.
- Remember the molar ratio of a balanced equation gives you the ratio of the amounts of each substance in the reaction.
The document Amounts of Substances in Equations | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
73 videos|87 docs|21 tests
|
Related Searches