Aromaticity - General Organic Chemistry | Organic Chemistry PDF Download
| Table of contents |

|
| Introduction |

|
| Huckel rule’s |

|
| Annulene |

|
| Bicyclic & Polycyclic Aromatic System |

|
| Craig's Rule |

|
Introduction
The factor which explains the extra stability of cyclic molecule is called ‘aromaticity’. In aromatic compound, resonance increases the stability of the molecule, therefore, energy is called resonance energy while in anti-aromatic compound, resonance decreases the stability of the compound, therefore, energy is called destabilization energy.
Huckel rule’s
For a compound to be aromatic, following conditions must be followed
(i) Compound must be cyclic & planar or nearly planar.
(ii) It must show resonance & delocalization.
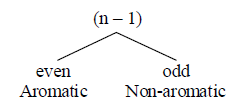
(iii) It obeys [4n + 2] π electron rule: n is called Huckel’s number or aromaticity index
Aromaticity index [n] = 0, 1, 2, 3….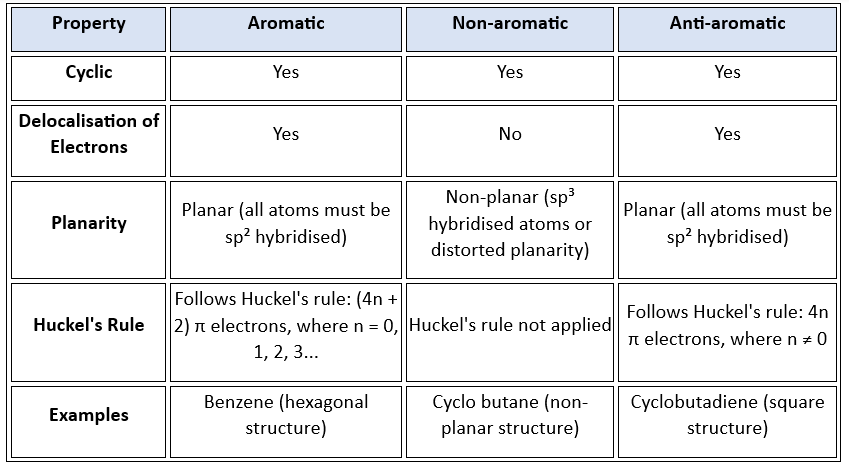
Homoaromatic molecules are molecules which are form by passing one or more than one sp3 carbon atom in the ring through delocalization of π-electron and it become aromatic in nature.
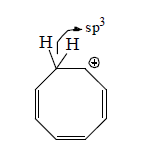
Stability order: Aromatic > Homoaromatic > Nonaromatic > Antiaromatic.
Number of π -electron in cyclic and a cyclic conjugated system
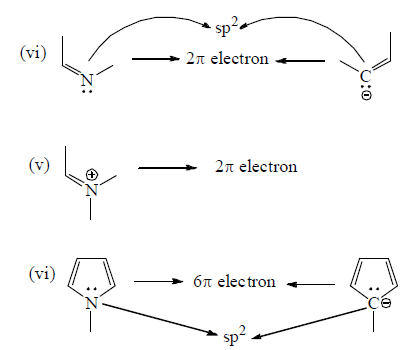
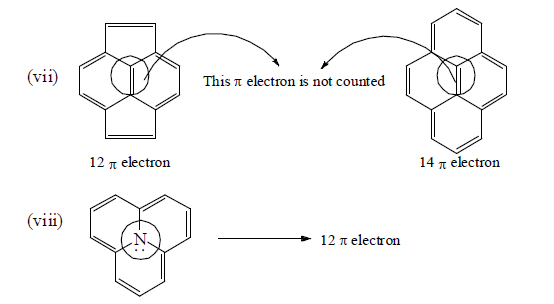
How To Apply Aromaticity Rule:
1. Aromaticity in three member ring.
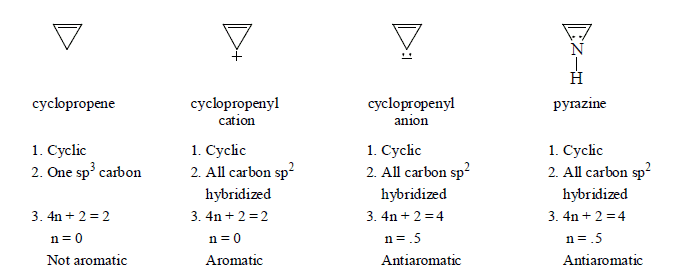
2. Aromaticity in four membered ring.
3. Aromaticity in five membered ring:
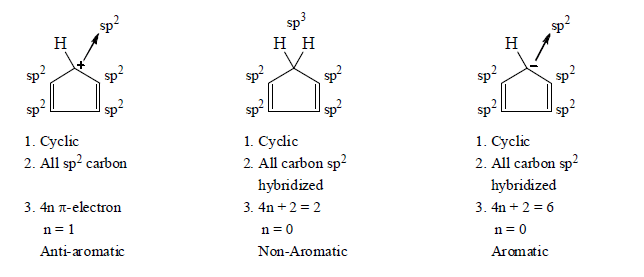
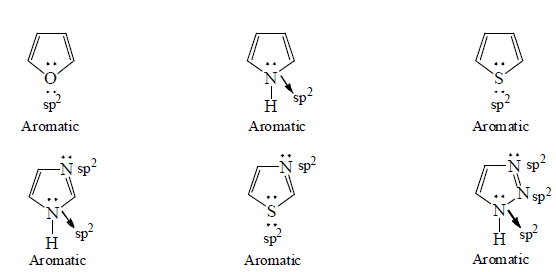
4. Aromaticity in six membered ring:
5. Aromaticity in seven membered ring:
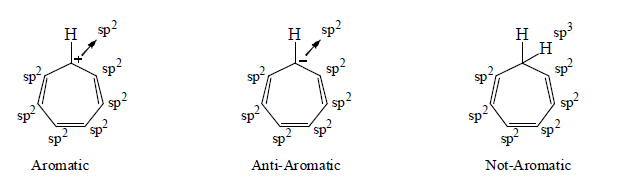
6. Aromaticity in eight membered ring:
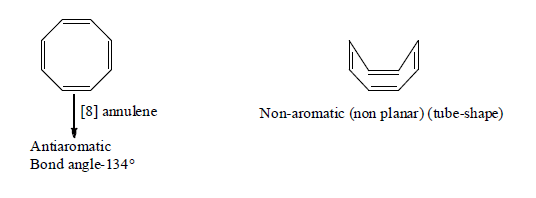
7. Non-Benzenoid aromatics Compounds: Annulenes
The monocyclic conjugated system of the general formula (CH)2n have been called annulenes, the number of carbon atoms inthe ring being denoted by a prefixed of breackts. In this system of nomenclature, benzene is [6] annulene and COT is [8] annulene. Since the carbon atoms occur as donded pair, an annulene must have an even of carbons. The annulenes can be grouped into two series.
(a) Those in which m is odd having (4n + 2) π -electrons.
(b) Those in which m is even having (4n) π -electrons.
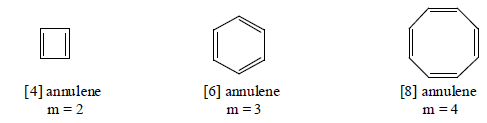
Here we will discuss only those anulenes which have (4n + 2) π-electrons and aromatic in character.
Annulene
Annulene can exist in three isomeric forms: di-trans--[10] annulene (1), all-cis-[10] annulene (2) and mono-trans-[10] annulesn (3).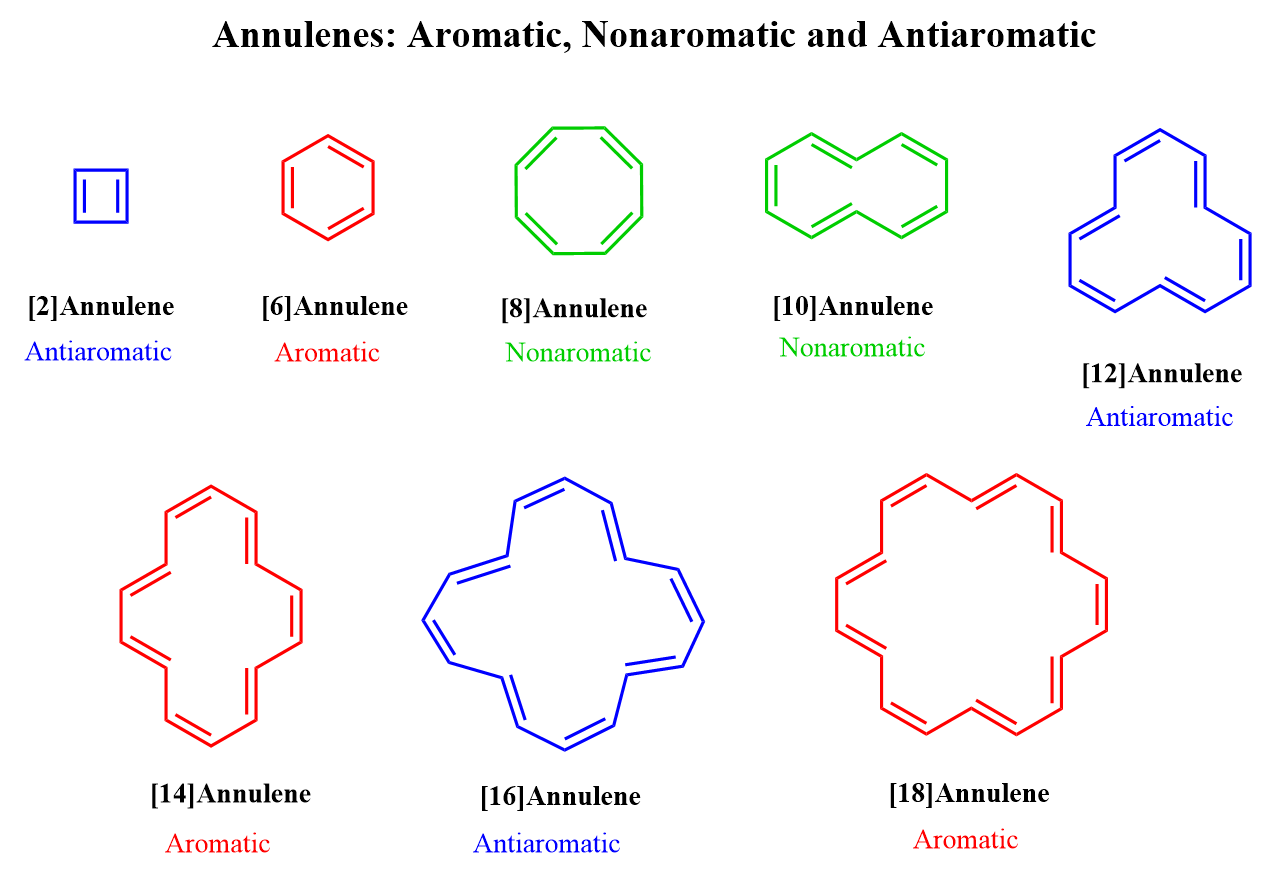
Althrough these three isomers have (4n + 2) π -electrons, they are not aromatic in character. All the three isomers are non-planar. In di-trans-[10] annulene two internal hydrogends prevent the molecule from assuming a planar configuration. Replacing two interacting hydrogens in di-trans-[10] annulene gives 1, 6-methano [10] annulene (4) in which the transannular interaction are removed. The NMR spectrum of (4) show an AB’BB’ system for the ring protons at low field [δ - 0.52] . This indicates that compound (4) is diatropic molecule and hence is aromatic.
[14] ANNULENE
[14] Annulene (cyclotetradecaheptaene) (5) follows the Huckel (4n + 2) π rule; but models and scale drawing indicate that the four inner hydrogens overlap so that a planar molecule is unlike. X-ray studies show that althrough all carbon-carbon bonds are of equal length but molecule is not planar. [14] Annulene is a diatropic molecule. Its inner protons appears at δ0.00 and outer protons appear at δ7.6 . Thus [14] annulene is aromatic althrough it is not planar.
Althrough [14] annulene is aromatic, the overlapping of the van der Waals radii of the four inner hydrogens reduces it aromaticity. thus, [14] annulene is not very stable. It is completely destroyed by light and air within 24 hrs. It does not give substitution reactions.
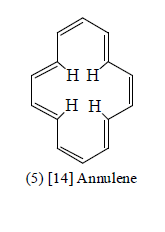
A variety of 1.6 : 8, 13 bridged [14] annulenes have been prepared with both carbon and heteroatom bridges. syn- 1, 6:8, 13-Bisoxio [14] annulene (6) is a red crystalline, thermally stable aromatic comopund. Syn-1, 6-methano-8, 13-oxiodo (7) is also diatropic in nature.

[18] Annulene (Cyclooctadecanonaene)
[18] Annulene (8) confirms the Huckel rule and, provided the molecule can assume nearly a planar conformation, should exhibit aromaticity. Molecular models and scale drawing indicate that the six inner hydrogens overlap slightly, but that this overlap is not so extreme as to exclude a reasonably planar structure.
A three dimensional X-ray crystallographic analysis of [18] annulene showered that it was a near planar molecule with non-alternate bonds of two type six cisolid (141.9 pm) and twelve transoid (138.2 pm). The resonance energy of the comopund is 37 kal/mole. The NMR Spectrum of the compuond showred that it is a diatroic molecule: the twelve outer protons appear at about δ9.0 and the six inter protons at δ-3.0.
It is stable in crystalline state but decomposes in solution at room temperature. [20]
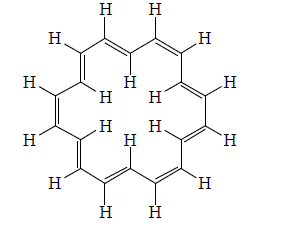
[20]Annulene:
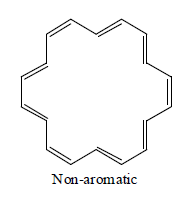
[22] Annulene
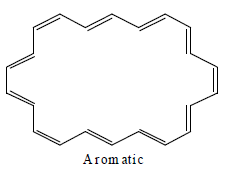
Annulene are found to be non-aromatic as per Huckel rule because their planarity are not justified therefore they are considered to be non-aromatic.
Bicyclic & Polycyclic Aromatic System
Bicyclic and polycyclic aromatic systems consist of two or more fused rings that satisfy aromaticity criteria, including cyclic, planar structures with delocalized π-electrons following Huckel's rule (4n+2).
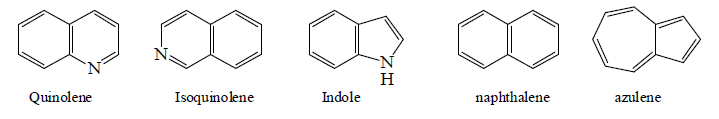
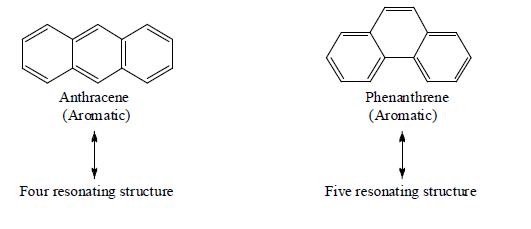
Examples: Naphthalene (10 π-electrons, two fused benzene rings), anthracene (14 π-electrons, three linearly fused rings), and phenanthrene (three angularly fused rings). These systems are stable and widely used in dyes, organic synthesis, and materials science. Other notable systems include indole (benzene fused with pyrrole) and coronene (six fused rings), known for their unique properties in electronics and biosynthesis.
Bicyclic Aromatic System
Tricyclic
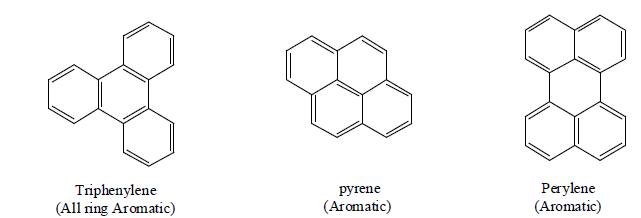
Tetracyclic
Craig's Rule
Craig's Rule is a guideline used in the field of chemistry to predict the planarity or non-planarity of heteroaromatic systems. It focuses on the interplay of lone pairs on heteroatoms within cyclic systems to determine whether the molecule will be planar and thus potentially aromatic.
Condition:
Single Lone Pair Involvement: At most one lone pair per heteroatom can participate in the delocalized π-electron system.
Delocalized Lone Pair: At least one lone pair on the heteroatom must be delocalized into the π-system for aromaticity.
Hybridization: The heteroatom must be sp² hybridized to allow effective overlap of its p-orbital with the π-system.
Avoiding Repulsion: Significant electronic repulsion between lone pairs (on the same or neighboring atoms) must be avoided to maintain planarity.
Cyclic Structure: The compound must be cyclic, ensuring a continuous conjugated π-electron system.
Planarity: The molecule should remain planar to allow delocalization of π-electrons.
Huckel’s Rule: The system must satisfy Huckel’s rule for aromaticity: (4n+2) π-electrons (where n=0,1,2...).
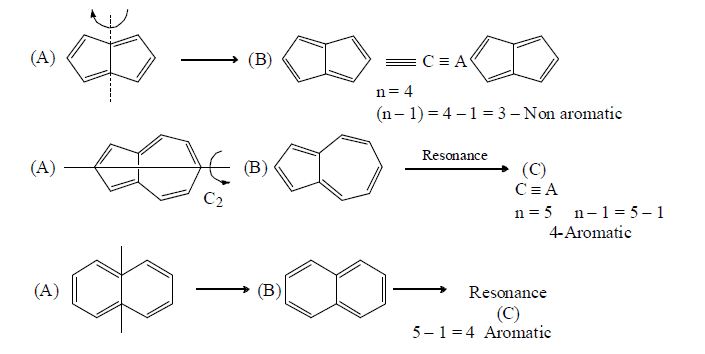
Annulation effect: In aromaticity the delocalisation should be restricted to single ring in polycyclic compounds. As the delocalisation passes from one ring to another it loses its extent of aromaticity. This is called annulation effect.
|
44 videos|102 docs|52 tests
|
FAQs on Aromaticity - General Organic Chemistry - Organic Chemistry
| 1. What is Huckel's rule and how is it applied to determine aromaticity in compounds? |  |
| 2. What are annulenes and how do they relate to aromatic compounds? |  |
| 3. Can you explain the difference between bicyclic and polycyclic aromatic systems? |  |
| 4. What is Craig's rule and how does it apply to the stability of aromatic compounds? |  |
| 5. Why is aromaticity important in organic chemistry? |  |
















