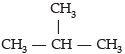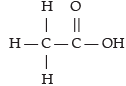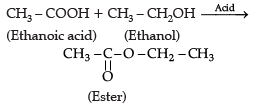Q1:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): Most of the carbon compounds are good conductors of electricity.
Reason (R): They do not dissociate to form ions and remain as molecules.
Explanation
Carbon compounds are mainly poor conductors of electricity.
Report a problem
Q2:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): Iso- butane is the isomer of C4H10.
Reason (R): Iso-butane has four C and ten-H atoms.
Explanation
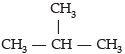
is the structural isomer of butane.
Report a problem
Q3:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself:Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: Following are the structural isomers of butane.
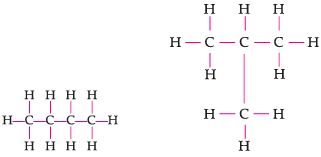
Reason: Structural isomers have the same molecular formula but they differ in their structures.
Explanation
Isomers are defined as those compounds that possess the same molecular formula but different structural arrangement. Butane has the molecular formula C4H10. Therefore, the structural isomers of butane will be n-butane and iso-butane.
Report a problem
Q4:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself:Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): In a homologous series of alcohols, the formula for the second member is C2H5OH and the third member is C3H7OH.
Reason (R): The difference between the molecular masses of the two consecutive members of a homologous series is 144u.
Explanation
In a homologous series of alcohols, the formula for the second member is C2H5OH and the third member is C3H7OH. The difference between the molecular masses of the two consecutive members of a homologous series is 14 because a -CH2 group has a molecular mass of 14 amu.
Hence, the correct answer is the assertion is true and Reason is false.
Report a problem
Q5:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): Third member of alkane is propane (C3H8)
Reason (R): It is obtained from general formula CnH2n + 2
Explanation
C3H8 can be obtained from the general formula, CnH2n + 2.
Report a problem
Q6:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): Following are the members of a homologous series : CH3OH, CH3CH2OH, CH3CH2CH2OH
Reason (R): A series of compounds with the same functional group but differing by ––CH2 – unit is called a homologous series.
Explanation
A homologous series is a group of compounds that differ by a constant unit, generally a CH2 group.
Report a problem
Q7:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): CH3Cl is obtained from CH4 by the action of Cl2 in the presence of sunlight.
Reason (R): It is obtained by an additional reaction.
Explanation
CH3Cl is obtained from CH4 by substitution reaction by the action of Cl2 in the presence of sunlight.
Report a problem
Q8:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): In esterification, carboxylic acid and alcohol react in the presence of acid to give ester.
Reason (R): Esterification is the reverse of saponification.
Explanation
In esterification, RCOOH, – H is replaced by – R' of R'OH in the presence of acid to form RCOOR'.
Report a problem
Q9:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself: Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:Assertion (A): Acetic acid has six single bonds and one double bond.
Reason (R): It is an unsaturated organic compound.
Explanation
Acetic acid has a structure which has six single bonds and only one double bond. It is an unsaturated organic compound.
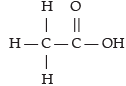
Report a problem
Q10:
Question for Assertion & Reason Type Questions: Carbon and its compounds
Try yourself:Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): Esterification is a process in which a sweet smelling substance is produced.
Reason (R): When esters react with sodium hydroxide an alcohol and sodium salt of carboxylic acid are obtained.
Explanation
Esterification is a reaction in which alcohol like ethanol reacts with carboxylic acids to form esters and water in the presence of sulphuric acid. Esters are generally sweet smelling substances
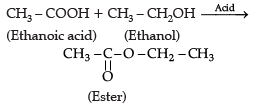
Report a problem