Atomic and Nuclear Physics | General Awareness for SSC CGL PDF Download
Introduction
The study of atomic structure, radioactivity, and modern applications of electromagnetic radiation provides insight into the fundamental aspects of matter and energy. This comprehensive overview covers the core principles of atomic particles, radioactive decay, and various technological applications that utilize these concepts.
Atomic Structure
- Atom is the smallest part of any matter which carries all the chemical and physical properties as the matter.
- All the matters in the universe are made up of atoms, which in turn are made up of mainly three particles electron, proton and the neutron.
Composition of the Nucleus
- Nucleus consists of protons and neutrons. Protons provide positive charge and protons-neutrons combindly provide entire mass to a nucleus.
- Proton was discovered by Rutherford when he bombard the nitrogen nuclei with α-particles.

- The charge on a proton is + 1.6 × 10−19 coulomb and its mass is 1.672 × 10−27 kg.
- Neutron was discovered by J. Chadwick when he bombard beryllium with α-particles
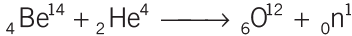
- Neutron is a neutral particle and its mass is 1.675 × 10−27 kg.
- The number of protons in the nucleus of an atom of the element, is called atomic number (Z ) of the element.
- The total number of protons and neutrons present inside the nucleus of an atom of the element, is called mass number (A) of the element. The atoms of an element having same atomic number but different mass numbers, are called isotopes.
Examples 1H1, 1H2 , 1H3 are isotopes of hydrogen. - The atoms of different elements having same mass number but different atomic numbers, are called isobars.
Examples 1H3 , 2He3 and 11Na22 , 10Ne22 are isobars. - The atoms of different elements having different atomic numbers and different mass numbers but having same number of neutrons, are called isotones.
Examples 1H3 , 2He4 and 6C14, 8O16 are isotones.
Radioactivity
The spontaneous process by which a nucleus changes its state with the emission of some particle or radiation is called radioactivity.
Types of Radioactive Decay
In nature, three types of radioactive decay occurs
(i) α-decay
(ii) β-decay
(iii) γ-decay
- This phenomenon was discovered by a French Physicist Henry Becquerel in 1896. When an α-particle is emitted by a nucleus, its atomic number decreases by 2 and mass number decreases by 4.

- When β-particle is emitted by a nucleus, its atomic number increases by 1 and mass numbers remains unchanged.

- When γ-rays are emitted by a nucleus. Its atomic number and mass remain unchanged.

Where, (*) stands for excited atom.
- In nature three types of radioactive decay occurs.
Radioactive Decay Law
- The rate of disintegration of radioactive atoms at any instant is directly proportional to the number of radioactive atoms present in the sample at that instant.
- Rate of disintegration
 where, λ is the decay constant.
where, λ is the decay constant.
- The number of atoms present undecayed in the sample at any instant N = N0e–λt
where, N0 = number of atoms at time t = 0
N = number of atoms at time t
Important Terms Related to Radioactivity
- The time in which the half number of atoms present initially in any sample decays, is called half-life (T) of that radioactive element.
- Relation between half-life and disintegration constant

- Average life or mean life (τ) of a radioactive element is the ratio of total life time of all the atoms and total number of atoms present initially in the sample.
- Relation between average life and decay constant τ = l/λ
- Relation between half-life and average life τ = 1.44 T
- The number of atoms left undecayed after n half- lives is given by N = N0 (1/2)n
where, n = t/T
Here, t = total time - The activity of a radioactive element is equal to its rate of disintegration.
Activity, R = (-dn/dt) - Activity of the sample after time t is given by R = R0 e−λt
- Its SI unit is Becquerel (Bq). Its other units are Curie and Rutherford.
1 curie = 3 .7 × 1010 decay/s
1 rutherford = 106 decay/s
Nuclear Fission
- The process of the splitting of a heavy nucleus into two or more lighter nuclei, is called nuclear fission.
- When a slow moving neutron strikes with uranium nucleus (92U235) , it splits into 56Ba141 and 36Kr92 along with three neutrons and a lot of energy.

- If the particle starting the nuclear fission reaction is produced as a product and further takes part in the nuclear fission reaction, then a chain of fission reaction started, which is called nuclear chain reaction.
- Nuclear chain reaction are of two types
– Controlled chain reaction
– Uncontrolled chain reaction
Nuclear Reactor
The working of a nuclear reactor is based on controlled chain reaction.
The main parts of a nuclear reactor are as follows
- Fuel Fissionable materials like 92U235, 92U238, 94Pu239 are used as fuel.
- Moderator Heavy water, graphite and beryllium oxide are used to slower down fast moving neutrons.
- Coolant The cold water, liquid oxygen etc., are used to remove heat generated in the fission process.
- Control rods Cadmium or boron rods are good absorber of neutrons and therefore, used to control the fission reaction.
- Atom bomb working is based on uncontrolled chain reaction.
- The nuclear reactors in which energy is produced by the fission of U235 by slow moving neutrons, are called thermal reactors.
- The nuclear reactors in which energy is produced by the fission of Pu239 or U233 , are called breeder reactors.
- Pu239 is produced from U238 and U233 is produced from Th235
Working of a Nuclear Reactor
When reactor is to be started, the cadmium rods are pulled out. Any neutron present in the reactor starts fission reaction of U235 . The neutrons produced in fission process further takes part in the fission process and a chain reaction of fission started. The rate of reaction is controlled by pressing the cadmium rods inside the reactor. A large amount of heat is produced in fission process which is continuously carried out with the supply of cool water.
Nuclear Fusion
- The process of combining of two lighter nuclei to form one heavy nucleus, is called nuclear fusion. In this process, a large amount of energy is released.
- Nuclear fusion takes place at very high temperature approximately about 107 K and at very high pressure 106 atmosphere.
- Hydrogen bomb is based on nuclear fusion and it is more destructive than an atom bomb. The source of the sun’s energy is the nuclear fusion process taking place at the sun.
Dangers of Nuclear Leakage
- Nuclear power plants should be installed far away from populated areas. Its building or structure should be made in such a way that it can bear the earthquake. Proper supply of coolant (normal water) should be available in normal working conditions or at the time of any accident.
- A nuclear disaster has thrilled Japan currently March 11, 2011. At Fukushima, nuclear power plants are out of work due to an earthquake. Radiations leakage is continuously contaminating water in the coastal areas and air in a wide range.
- The nuclear energy is a powerful source of energy but mishandling or carelessness can result as a disaster.
There are two types of radiations effect on human body- Somatic Effect These affects a human being which can be cured or cannot be cured but never transferred to the next generation.
- Genetic Effect These affects the genes of a person and therefore are transferred to the next generations.
Einstein’s Mass-Energy Relation
- According to Einstein, when ∆m mass is lost, the produced energy is given by
∆E = ∆mc2
where, c is the speed of light in vacuum. - The 12th part of the mass of a carbon atom (6C12), is called atomic mass unit.
1 amu = 1.66 × 10−27 kg = 931 meV - The minimum energy required to separate the nucleons upto an infinite distance from the nucleus, is called nuclear binding energy.
Nuclear binding energy per nucleon
Mass Defect
The difference between the sum of masses of all nucleons (M) and mass of the nucleus (m), is called mass defect.
Mass defect (∆m) = M − m
Fluorescence and Phosphorescence
- The absorption of energy by atoms, molecules etc followed by immediate emission of electromagnetic radiations when atoms or molecules return to their lower energy state. In fluorescence, the emission of light stops as the incident radiation is cut-off.
- On the other hand, the absorption of energy by atoms followed by emission of electromagnetic radiation is called phosphorescence.
Photoelectric Effect
The phenomenon of emission of electrons from a metal surface when light of suitable frequency is incident on it, is called photoelectric effect.
Einstein’s Photoelectric Equation
- Einstein explained photoelectric effect on the basis of Maxwell-Planck quantum theory. According to which light is emitted from a source in the form of packets or bundles of energy, called quanta or photon. The energy of each photon is given by
E = hν,
where, h = Planck’s constant
ν = frequency by incident light - Kinetic energy of emitted photoelectron is given by
EK = ν − φ
or
This is called Einstein’s photoelectric equation. - The electrons emitted during photoelectric current, are called photoelectrons.
- The minimum energy required to eject one photoelectron from a metal surface, is called its work function.
- The minimum frequency of incident light that can eject photoelectron from a metal surface, is called its threshold frequency. It is denoted by νo.
- The maximum wavelength of incident light that can eject photoelectron from a metal surface, is called its threshold wavelength. It is denoted by λmax.
 where, h = Planck’s constant
where, h = Planck’s constant - The negative potential given to the anode of a photoelectric cell for which photoelectric current becomes zero, is called the stopping potential or cut-off potential. It is denoted by V0.
- Maximum kinetic energy of photoelectrons is given by
Ek = eV0
where, e is the charge of an electron. - Cathode Rays These are formed by electrons emitted in a discharge tube when the pressure falls to about 10−4 mm of mercury.
- These rays travel in straight lines and deflected by electric and magnetic fields, can produce chemical changes.
- Positive Rays These are moving (in straight line) positive ions of the gas filled in the discharge tube and having mass nearly equal to the mass of the atoms of gas. These rays are deflected in magnetic field and can effect photographic plates and produce fluorescence and phosphorescence.
Laws of Photoelectric Effect
- The rate of emission of photoelectrons from a metal surface is directly proportional to the intensity of incident light.
- The maximum kinetic energy of photoelectrons does not depend on the intensity of incident light.
- Maximum kinetic energy of emitted photoelectrons increases with increase in frequency of incident light.
- If the frequency of incident light is below, than a certain minimum value called threshold frequency then no emission of photoelectrons takes place from the metal surface.
- There is no time lag between the incidence of light and emission of photoelectrons from the metal surface.
Electromagnetic Waves
- Electromagnetic waves are those waves in which electric and magnetic field vectors change sinusoidally and are perpendicular to each other as well as perpendicular to the direction of propagation of wave.
- These waves are produced by accelerating charge particles.
- These waves are transverse in nature.
- These waves do not require any medium for their propagation.
- The speed of electromagnetic waves in free space is given by
 where, ε0 = permittivity of free space and
where, ε0 = permittivity of free space and
µ0 = permeability of free space.
X-rays
- When cathode rays strike on a heavy metal having high melting point, then a part of energy of cathode rays converts into a new type of rays called X-rays.
- X-rays are electromagnetic waves of wavelengths ranging from 0.1 Å to 100 Å and frequencies ranging from 1016 Hz to 1018 Hz.
- Soft X-rays have greater wavelength of the order of 4 Å and lower frequency.
- Hard X-rays have lower wavelength of the order of 1 Å and higher frequency.
- X-rays are produced in coolidge tube.
- The intensity of X-rays depends on the heating voltage or filament current.
- The kinetic energy of X-ray photons depends upon the voltage applied across the ends of coolidge tube.
- X-rays are used to detect fractures of bones, presence of bullet or stone in the body.
- X-rays are used to cure cancer like diseases.
- X-rays can be diffracted by crystal following the Bragg’s law given by
2d sin θ = n × λ where, n = 1, 2, 3…
where , d = spacing between crystal planes
θ = angle of diffraction. - X-rays are used to detect gold and other costly metals hidden in sealed parcels or human body.
- X-rays are used in study of crystal structure and for detection of pearls in oysters.
- Electromagnetic Spectrum An arranged array of electromagnetic radiation in the sequence of their wavelength or frequency, is called electromagnetic spectrum.
- Radio and micro waves are used in radio and TV communication.
Infrared rays are used
- to treat muscular strain
- for taking photographs in fog or smoke
- in green house to keep plants warm
- in weather forecasting through infrared photography
- in night vision apparatus.
Ultraviolet rays are used
- in the study of molecular structure
- in sterilising the surgical instruments
- in the detection by forged documents, finger prints
- in the water purification system to kill harmful micro organism in water.
Devices Based on Electromagnetic Radiations
- A breath analyser is a tool used by law enforcement to identify individuals driving under the influence of alcohol.
- Infrared radiation can travel through dry air but is blocked by water vapor. When infrared radiation is directed through the breath of someone who has consumed alcohol, the transmission is altered.
- Infrared photography is used at airports to detect individuals with H1N1 (swine flu). Infrared-sensitive photographic film displays various colors for different temperatures, and a thermographic scanner creates a TV-like image of the infrared radiation emitted by different bodies. People with fever have a higher body temperature compared to others, making them easier to spot in a crowd at the airport.
- A microwave oven cooks food using microwaves generated by a magnetron at a frequency of 2450 MHz. These microwaves are absorbed by water, sugar, fat, and certain other molecules in the food, causing them to vibrate and generate heat. Since microwaves are not absorbed by air, glass, or paper, these materials do not heat up, leading to reduced cooking times.
- Metallic utensils block microwaves and should not be used in a microwave oven.
- A CT scan (Computed Tomography Scan) is a medical imaging technique that uses tomography to diagnose internal bodily structures, such as tumors. This method involves placing X-ray beams and detectors around the patient to capture images from various angles around a single axis of rotation, resulting in a three-dimensional image.
- MRI (Magnetic Resonance Imaging) is a diagnostic tool used when X-rays, ultrasound, or CT scans are insufficient. It employs a strong magnetic field and radio frequency pulses to produce detailed images of organs like the heart, kidneys, liver, and pancreas, which can be viewed on a computer screen and saved to a CD or printed.
- A TV remote control contains an integrated circuit (IC) and other components. When a button is pressed, it emits infrared signals that are received by the TV’s electronic circuit to perform the desired function.
Radar
Radar stands for Radio Detection and Ranging. It is utilized to locate, guide, or identify objects such as aircraft, ships, and missiles. Radar works by sending continuous or pulsed radio waves toward an object and receiving the waves reflected back from it.
Uses of Radar
Radar is employed to detect and measure the position and distance of clouds. It is also used to explore and identify metal or oil reserves. Additionally, radar helps in detecting the outer layers of the atmosphere.
Maser
Maser stands for Microwave Amplification by Stimulated Emission of Radiation. It is a device that produces a strong source of coherent microwave radiation. Similar to lasers, masers operate through population inversion and stimulated emission.
Uses of Masers
Masers are used to accurately determine the position of artificial satellites, fighter jets, and unwanted missiles. They are also used in ocean water to transmit important messages.
|
464 videos|571 docs|394 tests
|
FAQs on Atomic and Nuclear Physics - General Awareness for SSC CGL
| 1. What is the composition of the nucleus? |  |
| 2. What is nuclear fission? |  |
| 3. What is nuclear fusion? |  |
| 4. What is the radioactive decay law? |  |
| 5. How does a nuclear reactor work? |  |





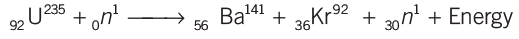


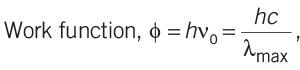 where, h = Planck’s constant
where, h = Planck’s constant where, ε0 = permittivity of free space and
where, ε0 = permittivity of free space and



















