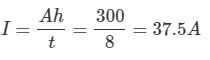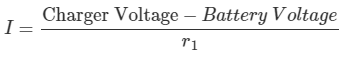Batteries, Fuel Cells & Corrosion | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| What is a Battery? |

|
| Primary Batteries |

|
| Secondary Batteries |

|
| Fuel Cells |

|
| Corrosion |

|
What is a Battery?
A battery is a device that stores chemical energy and converts it into electrical energy to provide a power source for various electronic devices and systems. Batteries are composed of one or more electrochemical cells, each of which contains two electrodes (an anode and a cathode) and an electrolyte that allows ions to move between the electrodes.

Usually, we use the term battery for a combination of a few cells that are similar in nature. A practical battery must have the following characteristics:
- It must be light in weight and compact in size.
- The cell or a battery must be able to give a constant voltage. Moreover, the voltage of the battery or the cell must not vary during use.
Types of Batteries
The batteries or the practical cells of the commercial values are mainly of two types. These are:- Primary cell/battery
- Secondary cell/battery.
Primary Batteries
The primary cells produce electricity by the virtue of a chemical reaction. Here the reaction occurs only in one direction. We can not reverse this phenomenon. As a result, these cells become dead over a period of time. You cannot reuse or recharge a primary cell. Some of the examples of primary cells are Daniel cell, Dry cell, and Mercury cell.
Daniel Cell
The Daniel cell has a copper vessel that contains a concentrated solution of copper sulphate. A porous pot containing dilute sulphuric acid is placed in the copper vessel containing copper sulphate solution. A zinc rod is dipped into dilute sulphuric acid. The zinc electrode acts as an anode, while the copper container acts as a cathode.
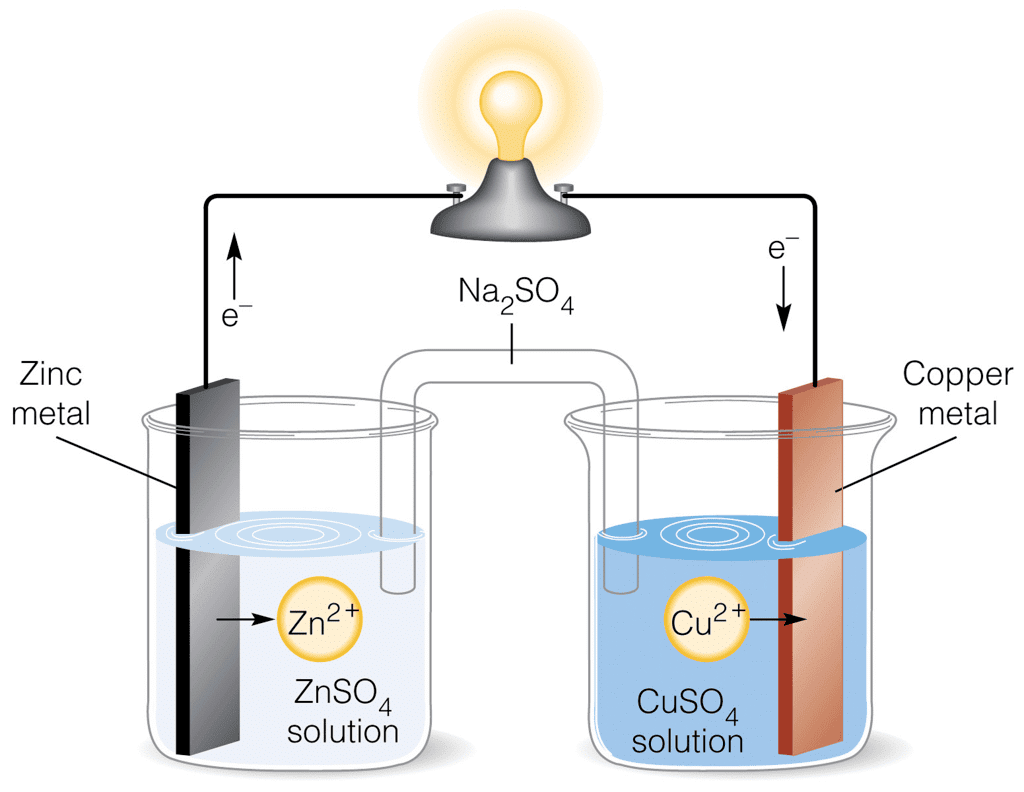
The reactions taking place in the cell are:
At anode: Zn(s) →Zn2+(aq) + 2e–
At cathode: Cu2+(aq) + 2e– →Cu(s)
Net cell reaction: Zn(s) + Cu2+(aq) →Cu(s) + Zn2+(aq)
The cell may be represented as,
| Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) (anode) (cathode) |
Daniel cell gives an emf of 1.1 V.
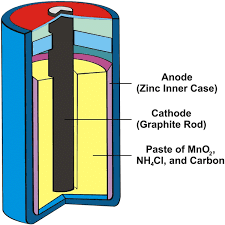
Dry Cell
A compact form of the LeClanche cell is the dry cell. It comprises of an outer container made of inc, which acts as an anode. The zinc content of the cell is lined from inside with porous insulating paper. The cathode is a carbon rod having a brass cap.
There is a space between the cathode and the anode which is filled with a mixture of MnO2 along with a thick paste of ammonium chloride, (NH4Cl), zinc chloride (ZnCl2), and charcoal. The lining of the porous paper prevents direct contact between the zinc container and the paste. It acts as a salt bridge. The cell is sealed from the top with pitch or wax.
 Dry Cells Reactions during discharge
Dry Cells Reactions during discharge
At anode: Zn(s) →Zn2++ 2e–
The Zn2+ ions migrate towards the carbon electrode (cathode).
At cathode: MnO2+ NH4++ e– → MnO (OH) + NH3MnO2
It acts as a depolarizer. The state of manganese is reduced from + 4 to + 3 in cathodic reaction. The ammonia molecules formed at the cathode react with Zn2+ ions coming from the anode, to form a complex ion Zn(NH3)42+. The complication of Zn2+ by NH3 molecules lowers the concentration of free Zn2+ and results in an increase in the voltage of the cell. A dry cell has a potential of about 1.5 V.
Are dry cells really dry?
In reality, the dry cells aren’t really dry. They have a wet paste of NH4Cl and ZnCl2. In reality, a dry cell will function only as long as the paste in the cell is moist. Moreover, you cannot recharge a dry cell. So, naturally, the dry cells do not have an indefinite life. This is because the NH4Cl paste is acidic in nature and it goes on corroding the zinc container even when it isn’t in use.
Mercury cell
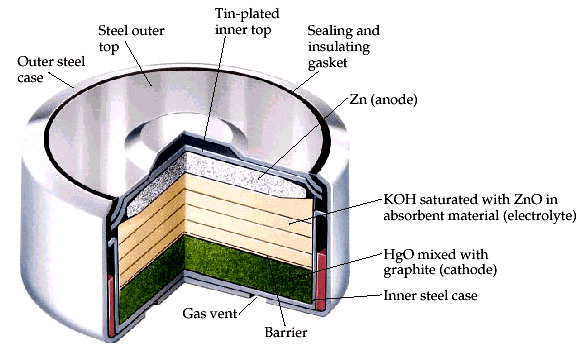
Mercury cell is recently introduced in the market. It offers a rather more stable voltage. The emf of the Mercury Cell is 1.35 V. Usually, the mercury cell is costlier. This is the reason, why they are used only in sophisticated instruments such as cameras, hearing aids, and watches, etc. Amalgamated zinc plate coated with a steel top plate acts as an anode in Mercury cell.
 Mercury cell
Mercury cell
A paste of Hg, HgO, and carbon powder acts as the cathode. It is placed in contact with the outer steel case. The electrolyte is a paste of KOH saturated with Zn(OH)2. An inert porous material carries this paste. The two electrodes are separated by an insulation seal of neoprene rubber. The reactions during discharge are,
At anode: Zn(Hg) + 2OH– →Zn (OH)2+2e–
At cathode: HgO + H2O + 2e– →Hg + 2OH–
Overall reaction: Zn(Hg) + HgO(s) →Zn(OH)2+ Hg(l)
Secondary Batteries
A secondary cell after use can be recharged by passing current through it in the opposite direction so that it can be used again. A good secondary cell can undergo a large number of discharging and charging cycles.
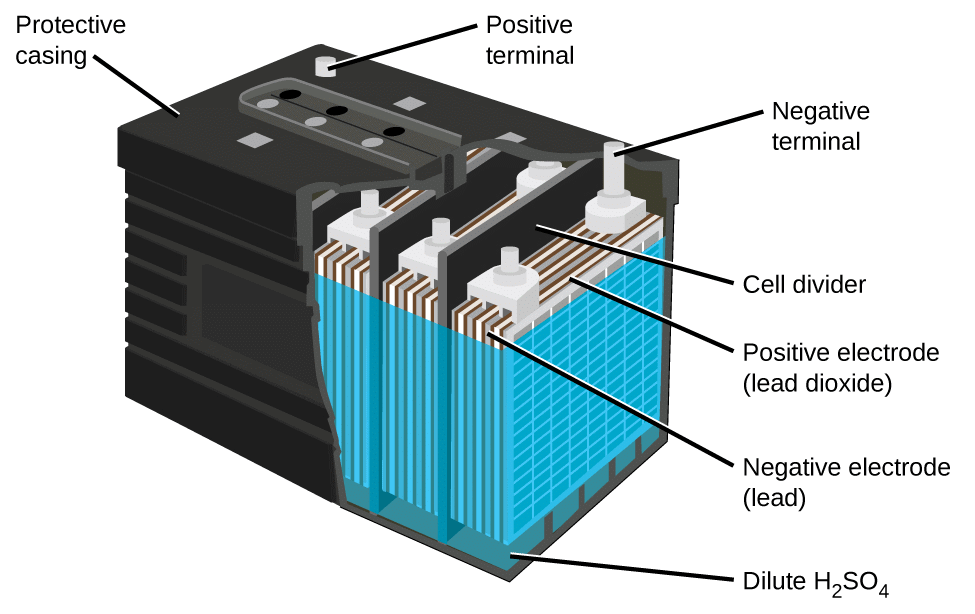
Lead Storage Cell:
The most important secondary cell is the lead storage battery commonly used in automobiles and invertors. It consists of a lead anode and a grid of lead packed with lead dioxide (PbO2 ) as cathode. A38% solution of sulphuric acid is used as an electrolyte.
The Cells reactions when the battery i sin use are given below:
Anode: Pb(s) + SO42-(aq) → PbSO4(s) + 2e–
Cathode: PbO2(s) + SO42-(aq) + 4H+(aq) + 2e- → PbSO4(s) + 2H2O(l)
i.e., overall cell reaction consisting of cathode and anode reactions is:Pb(s) + PbO2(s) + 2H2SO4(aq) ® 2PbSO4(s) + 2H2O(l)On charging the battery the reaction is reversed and PbSO4(s) on anode and cathode is converted intoPb and PbO2, respectii.e., overall cell reaction consisting of cathode and anode reactions is:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
On charging the battery the reaction is reversed and PbSO4(s) on anode and cathode is converted into Pb and PbO2, respectively
Lead-Acid Battery Construction: The lead-acid battery is the most commonly used type of storage battery and is well-known for its application in automobiles. The battery is made up of several cells, each of which consists of lead plates immersed in an electrolyte of dilute sulfuric acid. The voltage per cell is typically 2 V to 2.2 V. For a 6 V battery, three cells are connected in series, and for a 12 V battery, six cells are series-connected.
The construction of a lead-acid automobile type battery is shown below. The electrodes are lead-antimony alloy plates with a pattern of recesses so that they are in the form of grids.
 Plates:
Plates:
Lead oxide (termed active material) is pressed into the recesses of the plates. Each electrode consists of several plates connected in parallel with porous rubber separators in between, as illustrated in Figure 1 (b). This arrangement and the shape of the plates give the largest possible electrode surface area within the size limitations of the battery.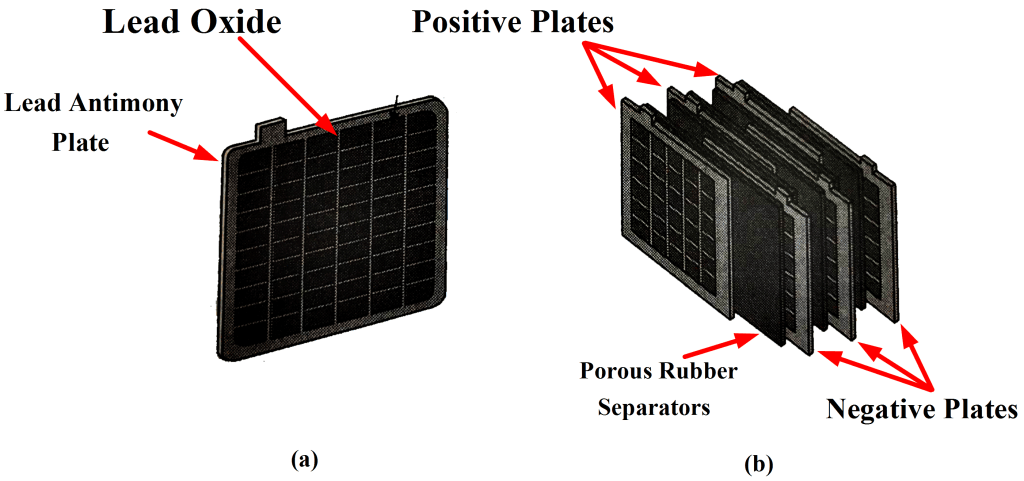
Rubber Case:
The complete 12 V battery, illustrated in Fig. 1, has an outer case of hard rubber. The case is divided into six sections for the six separate cells. Projections are provided on the inside at the bottom of the case to support the plates.
These projections ensure that the lower edges of the plates are normally well above the level of an active material that falls to the bottom of a cell. Such material can short out the positive and negative plates and render a cell useless.
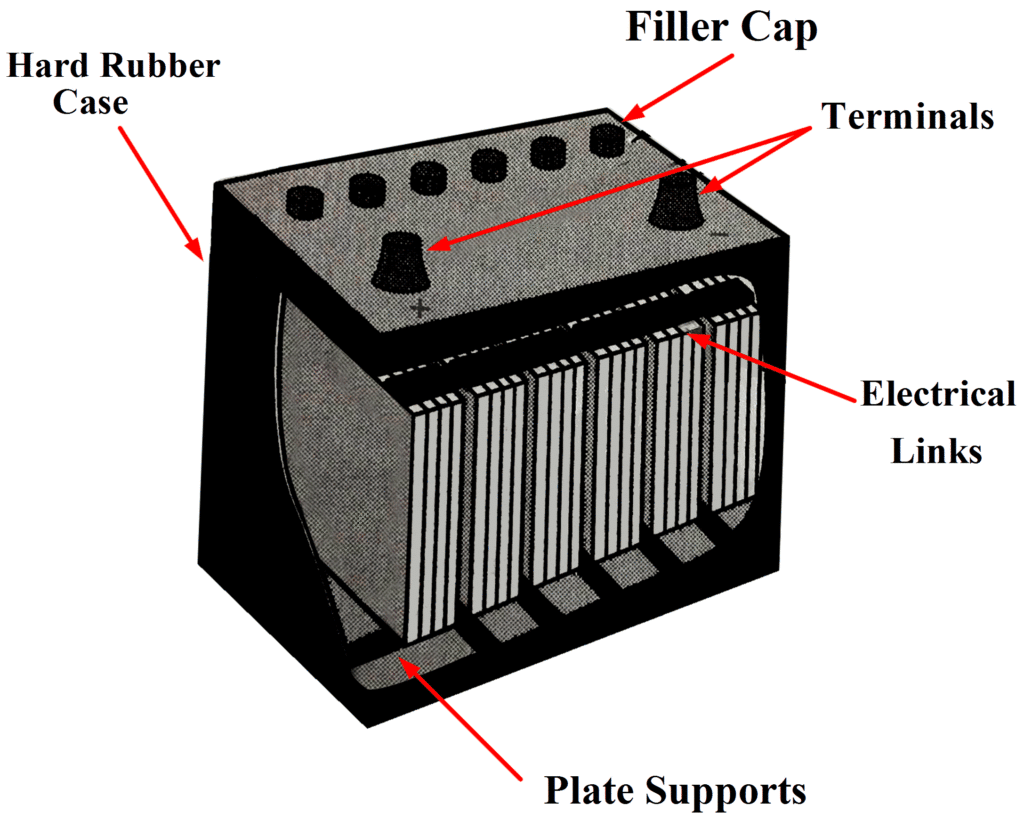
Every cell has a threaded filler cap with a small hole in its centre. The filler caps provide access for adding electrolyte, and the holes allow gases to be vented to the atmosphere.
Electrical Links:
Thick electrical links connect the cells in series, and hefty battery terminals are provided. Because the battery may be required to supply a very heavy current, it is important that the resistance of all electrical connections be very low to minimize voltage drops. A current of 250 A is not unusual for a battery driving an automobile starter.
Lead-Acid Battery Working:
When the lead-acid cell is charged, the lead oxide on the positive plates changes to lead peroxide, and that on the negative plates becomes a spongy or porous lead. In this condition, the positive plates are brown in color, and the negative plates are gray.
Lead-Acid Battery Discharging:
When the battery is discharging (i.e., supplying a current), atoms from the spongy lead on the negative plates combine with sulfate molecules to form lead sulfate and hydrogen. As always, electrons are left behind on the negative plates so that they maintain a negative potential.
- The hydrogen released in the electrolyte combines with the lead peroxide on the positive plate, removing electrons from the plate to keep a positive potential. The combination of lead peroxide and hydrogen at the positive electrode produces water and lead sulfate.
- The water dilutes the electrolyte, making it a weaker solution, and the lead sulfate that is produced at both positive and negative plates tends to fill the pores of the active material.
- Both these effects (dilution of the electrolyte and formation of lead sulfate) render each cell less efficient and eventually cause the battery output voltage to fall.
Lead-Acid Battery Recharging:
When the battery is recharged, a current (conventional direction) is made to flow into the positive electrode of each cell.
- This current causes the lead sulfate at the negative electrode to recombine with hydrogen ions, thus re-forming sulfuric acid in the electrolyte and Spongy lead on the negative plates.
- Also, the lead sulfate on the positive electrodes recombines with water to regenerate lead peroxide on the positive plates and sulfuric acid in the electrolyte. The final result of charging the cell is that the electrodes are re-formed and the electrolyte is returned to its original strength.
- With proper care a lead—acid battery is capable of sustaining a great many cycles of charge and discharge, giving satisfactory service for several years.
Ampere-Hour Rating of Lead-Acid Battery:
Typical ampere-hour ratings for 12 V lead-acid automobile batteries range from 100 Ah to 300 Ah. This is usually specified for an 8 h discharge time, and it defines the amount of energy that can be drawn from the battery until the voltage drops to about 1.7 V per cell.
For a 240 Ah rating, the battery could be expected to supply 30 A for an 8h period (see Figure 2). With greater load currents, the discharge time is obviously shorter. However, the ampere-hour rating is also likely to be reduced for a shorter discharge time because the battery is less efficient when supplying larger currents. Another method of rating a lead-acid battery is to define what its terminal voltage will be after about 5 s of supplying perhaps 250 A. This corresponds to the kind of load that a battery experiences in starting an automobile. It is important to avoid battery overloads that may demand excessive currents. Drawing a larger current than the battery is designed to supply may cause severe damage.
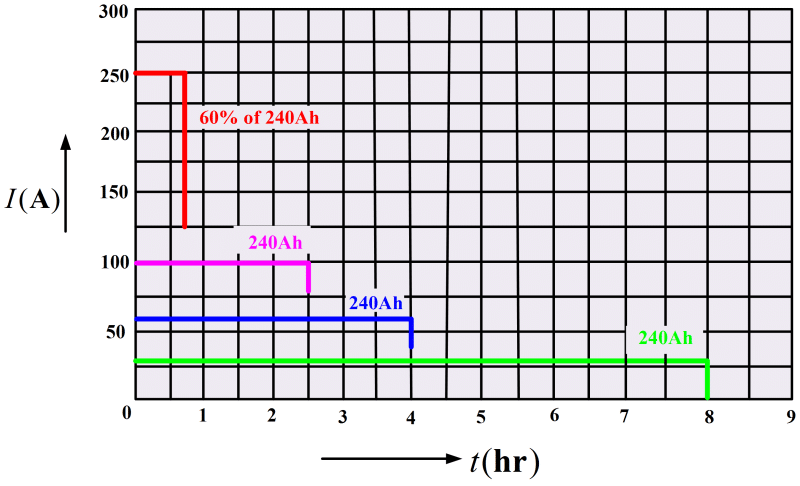
The rating of a battery is typically stated for temperatures around 25°C, and this must be revised for operation at lower temperatures. Because the chemical reactions occur more slowly at reduced temperatures, the available output current and voltage are less than at 25°C. Around -18°C a fully charged battery may be capable of delivering only 60% of its normal ampere-hour rating.
As the cell is discharged and the electrolyte becomes weaker, freezing of the electrolyte becomes more likely. A fully charged cell is less susceptible to freezing, but even a fully charged cell may fail when its temperature falls to about -21°C.
Example 1: A lead-acid battery has a rating of 300 Ah.
1. Determine how long the battery might be employed to supply 25 A.
2. If the battery rating is reduced to 100 Ah when supplying large currents, calculate how long it could be expected to supply 250 A.
3. Under very cold conditions the battery supplies only 60% of its normal rating. Find the length of time that it might continue to supply 250 A to the starter motor of an automobile.
Ans: a. From the following equation,
Ah Rating=I×t
Therefore
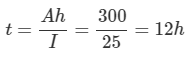
b. Time to supply 250 A current
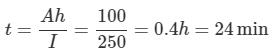
c. Time to supply 250 A at 60 % rating
Ah Rating = 60% of 100Ah = 60Ah
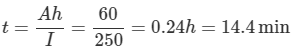
Example: What is the other name given to secondary cells?
Ans: In the secondary cells, the electrical energy is stored in the form of chemical energy. This is the reason, why they are also known as the storage cells or, accumulators.
Lead-Acid Battery Charging
When a battery is to be charged, a dc charging voltage must be applied to its terminals. The polarity of the charging voltage must be such that it causes current to flow into the battery, in opposition to the normal direction of the discharge current. This means that the positive output terminal of the battery charger must be connected to the positive terminal of the battery, and the charger negative terminal must be connected to the battery negative terminal. The arrangement is shown in Figure 3. The battery charger normally has a voltmeter and ammeter to monitor the charging voltage and current, and a control to adjust the rate of charge.
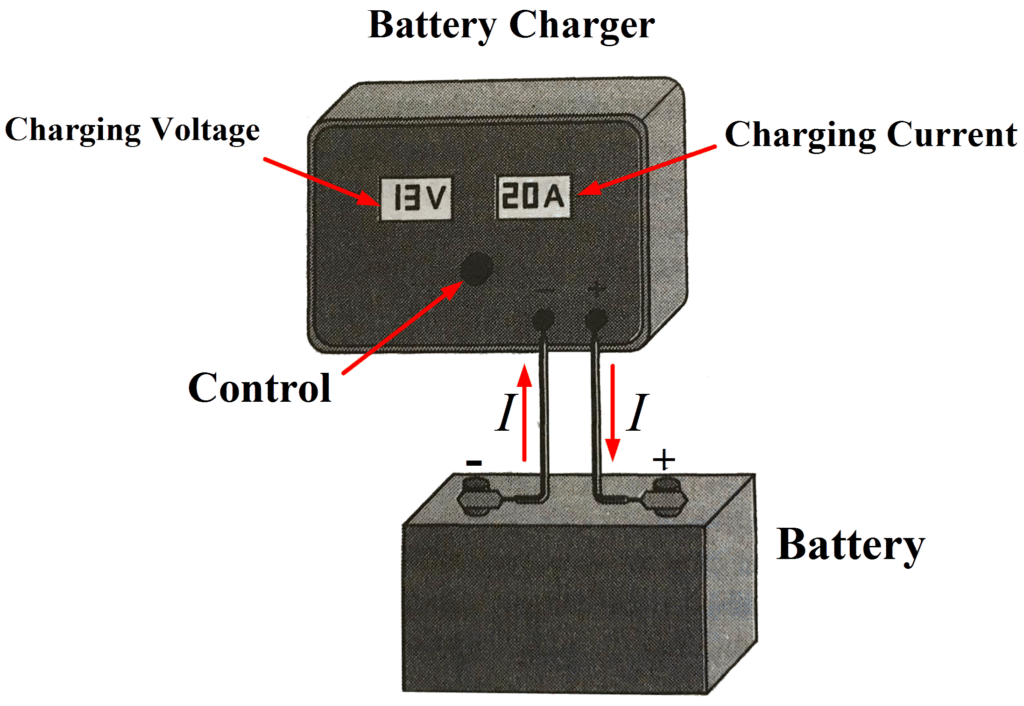 Fig 3: Lead-Acid Battery and Battery Charger Arrangement
Fig 3: Lead-Acid Battery and Battery Charger Arrangement
The output voltage of a battery charger must be greater than the battery voltage in order to cause current to flow into the battery positive terminal. The charging current depends on the difference between the battery voltage and the charging voltage and on the internal resistance of the battery.
A very large charging current is to be avoided because it could cause the battery to overheat, possibly resulting in warping of the lead plates. The maximum safe charging current is frequently taken as the maximum output current from the battery when discharging at its 8 h rate.
Example: A battery with a rating of 300 Ah is to be charged.
a. Determine a safe maximum charging current.
b. If the internal resistance of the battery is 0.008 Ω and its (discharged) terminal voltage is 11.5 V, calculate the initial output voltage level for the battery charger.
Ans:
a. Safe rate of charge at the 8h discharge rate:
b. Charging current:
Therefore,Charger Voltage = (I×r1) + Battery Voltage
= (37.5 × 0.008) + 11.5 = 11.8V
Lead-Acid Battery Specific Gravity:
When a lead-acid battery is in a nearly discharged condition, the electrolyte is in its weakest state. Conversely, the electrolyte is at its strongest (or greatest density) when the battery is fully charged. The density of electrolyte related to the density of water is termed its specific gravity.
The specific gravity of the electrolyte (measured by means of a hydrometer) is used as an indication of the state of charge of a lead-acid battery. Electrolyte with a specific gravity of 1100 to 1150 is 1.1 to 1.15 times as dense as water. At 1100 to 1150 the cell is completely discharged. When the specific gravity is 1280 to 1300, the cell may be assumed to be fully charged.
Care of Lead-Acid Batteries:
- The level of the electrolyte in each cell should be checked regularly, and distilled water added as necessary to keep the top of the plates covered by about 1 cm of liquid.
- Battery terminals should be kept clean and lightly coated with petroleum jelly to avoid corrosion.
- In cold weather, batteries should always be maintained in a nearly fully charged condition to avoid freezing.
- Lead-acid batteries should never be allowed to remain for a long period in a discharged state because lead sulfate could harden and permanently clog the pores of the electrodes.
- Before storing it for a long time the battery should be completely charged, then the electrolyte should be drained so that the battery is stored dry.
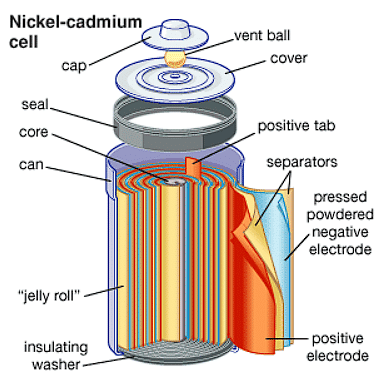
Nickel-cadmium cell :
Another important secondary cell is the nickel-cadmium cell which has longer life than the lead storage cell but more expensive to manufacture. We shall not go into details of working of the cell and the electrode reactions during charging and discharging
he overall reaction during discharge is:Cd (s) + 2Ni(OH)3(s) ® CdO(s) + 2Ni(OH)2 (s) + H2O(lThe overall reaction during discharge is:
Cd (s) + 2Ni(OH)3(s) →CdO(s) + 2Ni(OH)2 (s) + H2O(l)
 |
Download the notes
Batteries, Fuel Cells & Corrosion
|
Download as PDF |
Fuel Cells
Fuel cells are devices that convert chemical energy directly into electrical energy through an electrochemical reaction.
An advantage of voltaic cells is that they are small and portable, but their size is also a limitation. The amount of electric current produced is limited by the number of reagents contained in the cell.
When one of the reactants is completely consumed, the cell will no longer generate a current. Fuel cells avoid this limitation because the reactants (fuel and oxidant) can be supplied continuously to the cell from an external reservoir.
In a Hydrogen - Oxygen fuel cell figure, hydrogen is pumped onto the anode of the cell, and O2 (or air) is directed to the cathode where the following reactions occur.
Cathode, Reduction: O2(g) + 2H2O(l) + 4e- → 4OH-(aq) , Eº = 1.23 V
Anode, Oxidation: H2(g) → 2H (aq) + 2e- , Eº = 0V
Schematic diagram of a modern hydrogen-oxygen fuel cell. Commonly used electrolytes are NaOH solution, phosphoric acid, or solid oxides. A major limitation of any oxygen-consuming fuel cell is the slow rate of the reduction of this element at a cathode. The best cathode surfaces are usually made of platinum, which is a major cost factor in fuel cell design.
Corrosion
Corrosion is the process where materials, especially metals, deteriorate due to chemical reactions. The most common type is electrochemical corrosion, where metals lose electrons (oxidation) in the presence of an electron acceptor, called a depolarizer.
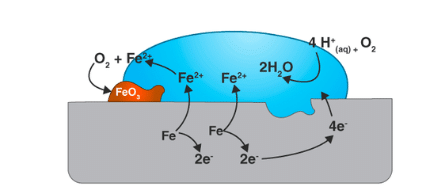
You can think of corrosion as metals naturally returning to their original ore state. The energy used to extract and refine metals is released as the metal corrodes.
In corrosion, the metal's oxidation (losing electrons) and reduction (gaining electrons) happen in different places. This separation is possible because metals conduct electricity, allowing electrons to move from the anodic (oxidation) areas to the cathodic (reduction) areas. Water is needed for this process to transport ions, but even a thin layer of moisture can be enough.
A corrosion system can be regarded as a short-circuited electrochemical cell:
Anode:
Fe(s) → Fe2+ (aq) + 2e- |
Cathode:
O2 + 2H2O + 4e- → 4OH- H+ + e- → ½H2(g) M2+ + 2e- → M(s) |
where M is a metal. Which parts of the metal serve as anodes and cathodes can depend on many factors, as can be seen from the irregular corrosion patterns that are commonly observed. Atoms in regions that have undergone stress, as might be produced by forming or machining, often tend to have higher free energies, and thus tend to become anodic.
Control of Corrosion
To prevent corrosion, we need to stop both the steps where metal loses electrons (anodic) and where it gains electrons (cathodic). The simplest way is to coat the metal with paint or another protective layer. However, these coatings might not cover everything perfectly, especially in areas with holes or threads.
A more advanced method is to apply a small negative charge to the metal. This makes it harder for the metal to lose electrons, which prevents the corrosion process from starting.
A more sophisticated approach is to apply a slight negative charge to the metal, thus making it more difficult for the reaction M →M2+ + 2e- to take place.
Sacrificial coatings
One way to protect metal from corrosion is by coating it with a more reactive metal. A common method is to cover steel with a thin layer of zinc, a process called galvanizing. Zinc corrodes more easily than iron, so it corrodes first. As the zinc layer corrodes, it releases electrons that flow into the iron, keeping it negatively charged and preventing it from corroding. This sacrificial zinc layer protects the steel underneath.
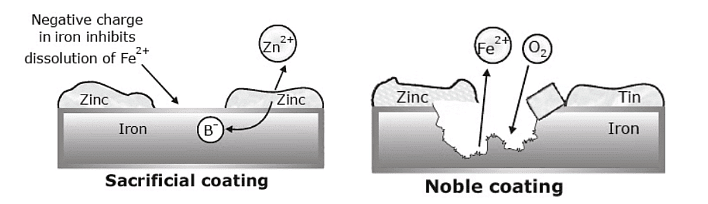
Plating iron with a less reactive metal, like tin, creates an interesting effect. For example, a tin-plated can is fine as long as the tin coating stays intact. However, if even a small part of the iron underneath is exposed to moisture, corrosion starts. The iron releases electrons that flow into the tin, making the iron more prone to corrosion. This means the tin ends up speeding up the corrosion of the iron. That's why tin cans left outside rust and fall apart quickly.
Cathodic Protection
A more advanced way to prevent metal corrosion is by keeping the metal negatively charged, which stops it from dissolving into positive ions. This technique is called cathodic protection because it keeps the whole metal surface in a cathodic (non-corroding) state.
There are two main ways to provide these electrons:
- Using an external direct current power source, which is common for protecting oil pipelines and other underground structures.
- Using a more active metal, like zinc or aluminum, that corrodes instead. This metal is placed in the ground near the structure to be protected.
|
108 videos|287 docs|122 tests
|
FAQs on Batteries, Fuel Cells & Corrosion - Chemistry Class 12 - NEET
| 1. What are primary batteries? |  |
| 2. What are secondary batteries? |  |
| 3. How do fuel cells work? |  |
| 4. How does corrosion affect batteries? |  |
| 5. What are some common applications of batteries, fuel cells, and corrosion? |  |