Behaviour of Gases | General Awareness for SSC CGL PDF Download
| Table of contents |

|
| Behavior of Gases |

|
| Gas Laws |

|
| Boyle’s Law |

|
| Charles’ Law |

|
| Gay-Lussac’s Law |

|
| Avogadro’s Law |

|
| Standard Gas Equation |

|
Behavior of Gases
 There are five main states of matter: solid, liquid, gas, plasma, and Bose-Einstein condensate. Among these, gases are particularly interesting and their behavior is relatively easy to study. The behavior of gases is described by various laws known as the gas laws, which detail the relationships between temperature, pressure, volume, and other properties. Here's an overview of these laws:
There are five main states of matter: solid, liquid, gas, plasma, and Bose-Einstein condensate. Among these, gases are particularly interesting and their behavior is relatively easy to study. The behavior of gases is described by various laws known as the gas laws, which detail the relationships between temperature, pressure, volume, and other properties. Here's an overview of these laws:
Gas Laws
In the periodic table, inert or noble gases are known for their low reactivity. Their behavior closely approximates that of an ideal gas, which has led to the formulation of the following gas laws based on experiments with these gases:
Boyle’s Law
- Suppose you have some Helium in a gas container at a low pressure and temperature.
- At a constant temperature, if you increase the volume of the container, the pressure of the gas will decrease. This is given by the Boyle’s law.
- This law states that at a constant temperature, the volume (V) of a given mass of gas is inversely proportional to its pressure (p). At constant temperature, Boyle’s law can be stated as:
V ∝ 1/P or VP = constant …(1) - The constant is a proportionality constant. Its values depend on the mass, temperature, and nature of the gas. If P1 and V1 are the initial values of pressure and volume of any gas and P2 and V2 are another set of values, then we can say that:
P1V1 = constant …(2) and P2V2 = constant …(3) - Since the mass, temperature, and nature of a gas are the same throughout, we say equation (2) and (3) represent the same quantity. Thus we have:
P1V1 = P2V2
Charles’ Law
Charles’ Law states that at constant pressure, the volume (V) of a given mass of gas is directly proportional to its absolute temperature (T).
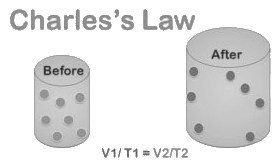
If V is the volume and T is the temperature of a gas at some constant pressure, then V ∝ T or V/T = constant. Following the same method as above, we can write:
V1/T1 = V2/T2
Gay-Lussac’s Law
Gay-Lussac’s Law, also known as Regnault’s Law, asserts that at constant volume (V), the pressure (P) of a given amount of gas is directly proportional to its absolute temperature (T). This relationship can be expressed as:
P1/T1 = P2/T2
Avogadro’s Law
- This law states that under the same temperature and pressure conditions, equal amounts of different gases contain the same number of molecules.
- So, if you have various gases that meet these conditions, they will occupy the same volume.
- For instance, at STP (Standard Temperature and Pressure) or NTP, where temperature (T) equals 273K and pressure (p) equals 1 atm, each gas with a volume of 22.4L holds NA = 6.023 x 1023 molecules.
- In simple terms, one mole of any gas at STP takes up a volume of 22.4L.
Standard Gas Equation
Gases that follow all gas laws regardless of pressure and temperature are known as ideal gases or perfect gases. Inert gases behave similarly to ideal gases when subjected to high temperatures and very low pressures.
The equation of state for a perfect gas can be represented as:
PV = nRT
where:
- P stands for pressure
- V represents volume
- T denotes absolute temperature
- R is the universal gas constant, equal to 8.31 J mol-1 K-1
- n indicates the number of moles of a gas
Real Gases
None of the gases found in nature follow gas laws for all temperature and pressure values.
Real gases, also known as "real gases," behave differently from ideal gases due to two main reasons:
- Real gas molecules are attracted to each other.
- Real gas molecules take up space.
Therefore, equations for such gases require adjustments.
Real Gas Equation or Van der Waal's Gas Equation
The equation of state for real gases can be expressed as:
- (P - a/V2)(V - b) = RT
- Here, 'a' and 'b' represent Van der Waal's constants.
|
478 videos|1421 docs|396 tests
|
FAQs on Behaviour of Gases - General Awareness for SSC CGL
| 1. What are the four gas laws that govern the behavior of gases? |  |
| 2. What is Boyle’s Law and how does it relate to the behavior of gases? |  |
| 3. How does Charles’ Law explain the behavior of gases? |  |
| 4. What does Gay-Lussac’s Law state and how does it impact the behavior of gases? |  |
| 5. How does Avogadro’s Law contribute to understanding the behavior of gases? |  |















