Benzene Electrophilic Substitution: Sulfonation, Nitration & Halogenation | Organic Chemistry PDF Download
Introduction
In 1825, Michael Faraday discovered that benzene is a colorless liquid. Cyclopentane is a compound with the molecular formula C6H6. This molecular formula clearly reflects an organic compound that is highly unsaturated. It is highly reactive as a result of this unsaturation. Since it does not react with additions, oxidations, and reductions like alkenes, it does not undergo these reactions. Benzene, for example, will not form carbon-carbon double bonds when reacting with Br, HCl or other reagents. Most of the time, the hydrogen atoms in benzene are replaced by another atom or radical during its reactions!
The aromatic group of compounds includes benzene. Due to its multiple aromas or odors, benzene and its derivatives were initially described as aromatic. Later, benzene was classified on the basis of its structural properties and chemical reactivity, not on its aroma. Now, aromatic compounds are defined as compounds that are extremely unsaturated and which are peculiarly stable in the presence of alkenes.
By analogy with alkane and alkene, today's arene is used to describe aromatic hydrocarbons. The parent arene of benzene is benzene. In the same way an alkyl group compound is represented by the symbol RJ, similarly, an aryl group containing its new atom or group is represented by ArJ.
Reactions of Benzene
Benzene is highly susceptible to electrophilic substitution reactions because, during addition reactions, it loses its aromaticity. Electrophiles are attracted to the delocalized electrons in benzene, and it is highly stable to electrophilic substitution due to its delocalized electrons.
Electrophilic substitution of benzene leads to the following three steps:
- The electrophile is created.
- An intermediate carbocation is removed of a proton.
Nitration
The reaction nitration of benzene is carried out by treating benzene with concentrated sulfuric acid and concentrated nitric acid at a temperature not exceeding 50°C. As the temperature increases, it becomes more likely that more than one nitro group, -NO2, will get substituted onto the ring, resulting in the formation of Nitrobenzene. A catalyst is involved in this reaction, which is sulfuric acid concentrated. NO+2 is the electrophile in this case, or the nitrogenium ion, or nitryl cation. Nitric acid and sulfuric acid react to form this compound.
When benzene and concentrated nitric acid react at 323-633 K in the presence of concentrated sulfuric acid, nitrobenzene is formed. Benzene nitration is the reaction responsible for making nitrobenzene.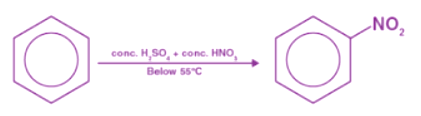 Step 1: The proton of sulphuric acid is accepted by nitric acid, which dissociates to form nitronium ions.
Step 1: The proton of sulphuric acid is accepted by nitric acid, which dissociates to form nitronium ions.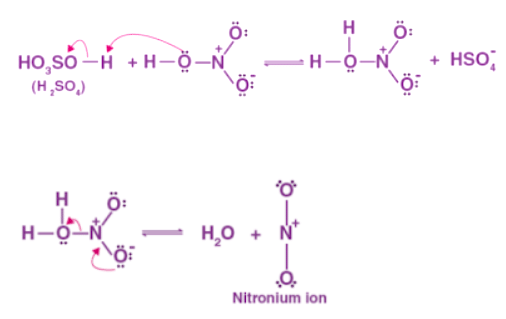 Step 2: This process generates an electrophilic nitronium ion, which then reacts with benzene to produce an arenium ion.
Step 2: This process generates an electrophilic nitronium ion, which then reacts with benzene to produce an arenium ion.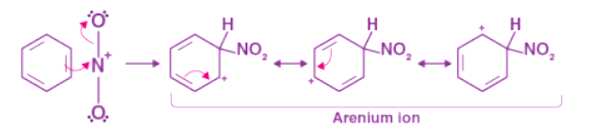 Step 3: As a Lewis base, nitrobenzene is formed by the arenium ion losing its proton.
Step 3: As a Lewis base, nitrobenzene is formed by the arenium ion losing its proton.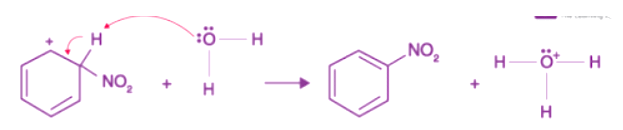
Sulphonation
Benzene and sulfuric acid undergo an electrophilic substitution reaction when they are sulfonated. The first way involves heating benzene at 40°C for several hours under the fumes of concentrated sulfuric acid. It is then converted into benzenesulphonate. Sulfur trioxide, SO3, is the electrophile here. Based on the kind of acid that is used, you can make sulfur trioxide electrophile in either of the two methods. Fumed sulfuric acid, H2S2O7, is a solution of SO3 in sulfuric acid, and it is thus a richer source of SO3. Concentrated sulfuric acid containing traces of SO3 may also produce it. Because of its highly polar nature and the fact that the sulfur atom carries a very strong charge, sulfur trioxide is electrophilic in nature. These electrons are attracted to the sulfur atom.
By heating fuming sulfuric acid (H2SO4, SO3) with benzene, benzenesulfonic acid is formed. Sulfuric acid fumes react with benzene reversibly.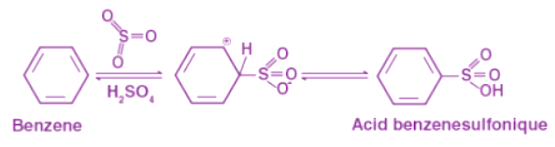 Due to its high electronegativity, sulphuric acid attracts an electron, which results in an electrophile. The benzene ring is attacked when this reaction occurs, resulting in benzenesulfonic acid.
Due to its high electronegativity, sulphuric acid attracts an electron, which results in an electrophile. The benzene ring is attacked when this reaction occurs, resulting in benzenesulfonic acid.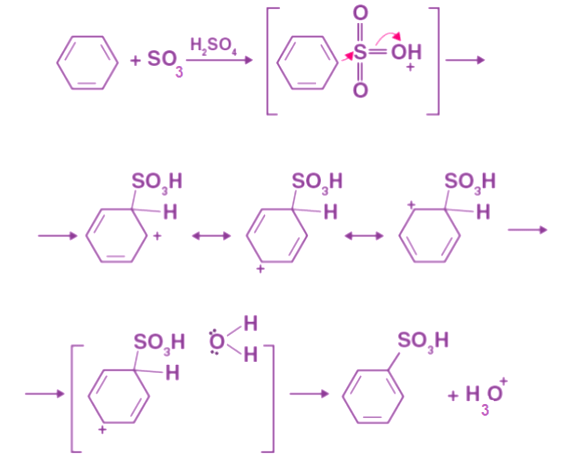
Halogenation
FeCl3 and FeBr3 react with benzene to form arylhalides when benzene is present. Halogenation of benzene is what this reaction is called.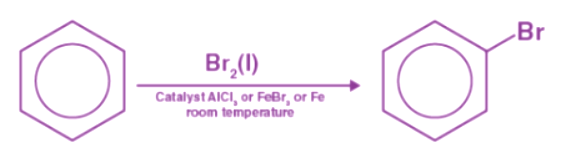 Step 1: When combined with the attacking reagent, FeBr3 contributes to the formation of electrophile bromine ions.
Step 1: When combined with the attacking reagent, FeBr3 contributes to the formation of electrophile bromine ions.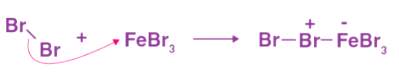 Step 2: Bromine acts as an electrophile in the reaction, forming arenium ions that are then converted to bromobenzene through reactions with benzene.
Step 2: Bromine acts as an electrophile in the reaction, forming arenium ions that are then converted to bromobenzene through reactions with benzene.
|
39 videos|92 docs|46 tests
|





















