Biological Effects of Radiation | Chemistry for ACT PDF Download
Introduction
Nuclear reactions generally do not have a direct impact on the valence electrons of an atom, except for electron capture which involves taking an electron from the lowest energy level orbital. As a result, they do not directly cause chemical alterations. However, the energetic particles and photons emitted during nuclear decay have the potential to indirectly bring about chemical changes in the surrounding matter. For example, an α particle, which is an ionized helium nucleus (He2+), can serve as a strong oxidizing agent. In the following section, we explain the interaction between radiation and matter, as well as some of the chemical and biological effects of radiation.
Ionizing versus Nonionizing Radiation
The impact of radiation on matter primarily relies on the energy of the radiation, which is determined by the nuclear decay reaction that generated it. Radiation categorized as nonionizing possesses relatively low energy. When it interacts with an atom within a molecule or an ion, most or all of its energy can be absorbed without causing any structural or chemical changes. Instead, the kinetic energy of the radiation is transferred to the atom or molecule it collides with, leading to rotational, vibrational, or increased translational motion. This energy can then be transferred to neighboring molecules or ions as heat, resulting in the warmth exhibited by many radioactive substances. For instance, highly radioactive elements like polonium have been utilized as heat sources in the US space program. As long as the nonionizing radiation intensity remains below the threshold that causes overheating, it is relatively harmless, and its effects can be counteracted through cooling.
In contrast, ionizing radiation is higher in energy, and some of its energy can be transferred to one or more atoms with which it collides as it passes through matter. If enough energy is transferred, electrons can be excited to very high energy levels, resulting in the formation of positively charged ions:
Molecules that have been ionized in this way are often highly reactive, and they can decompose or undergo other chemical changes that create a cascade of reactive molecules that can damage biological tissues and other materials (Figure 1). Because the energy of ionizing radiation is very high, we often report its energy in units such as megaelectronvolts (MeV) per particle: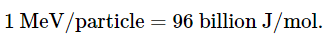
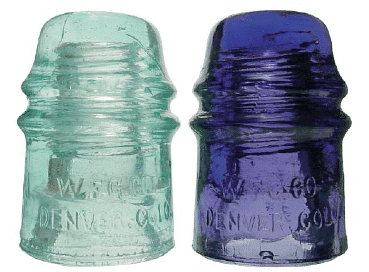
Figure 1: Radiation Damage: When high-energy particles emitted by radioactive decay interact with matter, they can break bonds or ionize molecules, resulting in changes in physical properties such as ductility or color. The glass electrical insulator on the left has not been exposed to radiation, but the insulator on the right has received intense radiation doses over a long period of time. Radiation damage changed the chemical structure of the glass, causing it to become bright blue.
The Effects of Ionizing Radiation on Matter
The effects of ionizing radiation depend on four factors:
- The type of radiation, which dictates how far it can penetrate into matter
- The energy of the individual particles or photons
- The number of particles or photons that strike a given area per unit time
- The chemical nature of the substance exposed to the radiation
Figure 2 illustrates the varying abilities of different forms of ionizing radiation to pass through biological tissues. Alpha (α) radiation, due to its high charge and mass, has a strong interaction with matter. Consequently, it does not penetrate deeply and can be effectively halted by materials such as paper, clothing, or skin. On the other hand, gamma (γ) rays, possessing no charge and negligible mass, have weak interactions with matter, allowing them to deeply penetrate most objects, including the human body. To fully block gamma rays, several inches of lead or over 12 inches of specialized concrete are required. Beta (β) particles fall in between alpha particles and gamma rays in terms of mass and charge, resulting in intermediate interaction with matter. Beta particles easily penetrate paper or skin but can be stopped by materials like wood or thin metal sheets.
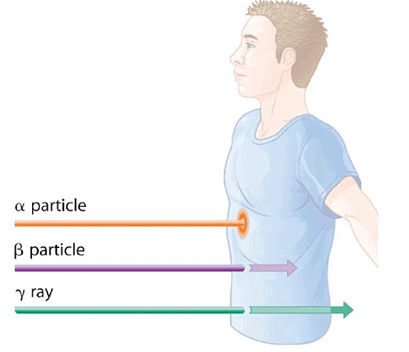
Figure 2: Depth of Penetration of Ionizing Radiation: The depth of penetration of alpha, beta, and gamma radiation varies with the particle. Because α particles interact strongly with matter, they do not penetrate deeply into the human body. In contrast, β particles do not interact as strongly with matter and penetrate more deeply. Gamma rays, which have no charge, are stopped by only very dense materials and can pass right through the human body without being absorbed.
When it comes to radiation originating from an external source, γ rays pose the greatest risk due to their high ability to penetrate matter. On the other hand, if the radiation source is within the body, alpha particles are the most harmful because the body's tissues absorb all of their energy. Therefore, the danger associated with radiation heavily depends on the type of radiation emitted and the level of exposure. This understanding enables scientists to handle numerous radioactive materials safely by taking precautions to prevent, for instance, inhalation of fine particulate dust containing alpha emitters. Table 1 provides a summary of certain characteristics of ionizing radiation.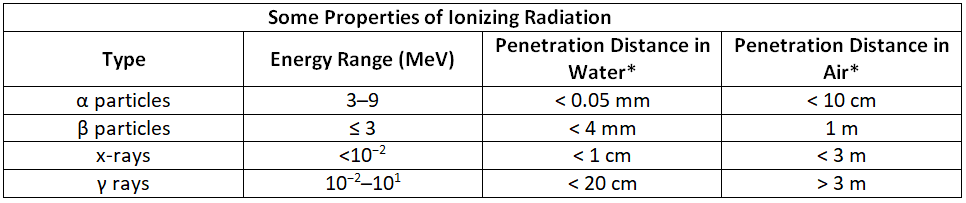
*Distance at which half of the radiation has been absorbed.
There are various methods available to quantify radiation exposure, or dose. The roentgen (R) is a unit used to measure the amount of energy absorbed by dry air, providing a quantitative measure of exposure. It is named after Wilhelm Röntgen, a German physicist who discovered X-rays and was awarded the Nobel Prize in Physics in 1901. Specifically, the roentgen is defined as the amount of radiation required to generate an electrical charge of 2.58 × 10−4 C in 1 kg of dry air. However, when it comes to assessing the impact on biological tissues, the relevant factor is the amount of energy absorbed by the tissues, not the air. The rad (radiation absorbed dose) is the most commonly used unit to measure the effects of radiation on biological tissue, with the gray (Gy) being its SI equivalent. The rad is defined as the amount of radiation that results in the absorption of 0.01 J of energy per kilogram of matter, while the gray is defined as the amount of radiation that leads to the absorption of 1 J of energy per kilogram.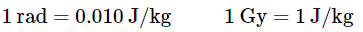 Therefore, if a person weighing 70 kg receives a dose of 1.0 rad throughout their entire body, they would absorb 0.010 J/70 kg = 1.4 × 10−4 J, which is equivalent to 0.14 mJ. To put this into perspective, 0.14 mJ is the amount of energy that would be transferred to your skin by a droplet of boiling water weighing 3.8 × 10−5 g. However, since the energy from the water droplet is dispersed over a relatively large area of tissue, it is harmless. In contrast, a radioactive particle transfers its energy to a single molecule, resembling a high-powered rifle bullet on the atomic scale.
Therefore, if a person weighing 70 kg receives a dose of 1.0 rad throughout their entire body, they would absorb 0.010 J/70 kg = 1.4 × 10−4 J, which is equivalent to 0.14 mJ. To put this into perspective, 0.14 mJ is the amount of energy that would be transferred to your skin by a droplet of boiling water weighing 3.8 × 10−5 g. However, since the energy from the water droplet is dispersed over a relatively large area of tissue, it is harmless. In contrast, a radioactive particle transfers its energy to a single molecule, resembling a high-powered rifle bullet on the atomic scale.
Due to the significantly higher mass and charge of α particles compared to β particles or γ rays, the difference in mass can be likened to being struck by a bowling ball instead of a table tennis ball moving at the same speed. Consequently, the amount of tissue damage caused by 1 rad of α particles is far greater than the damage caused by 1 rad of β particles or γ rays. To accurately describe the actual extent of tissue damage caused by a specific radiation dose, a unit known as the rem (roentgen equivalent in man) was introduced. The number of rems of radiation is calculated by multiplying the number of rads by the RBE (relative biological effectiveness) factor, which is 1 for β particles, γ rays, and x-rays, but approximately 20 for α particles. Given that radiation doses are typically very small, measurements are often reported in millirems (1 mrem = 10−3 rem).
Wilhelm Röntgen: Röntgen, who was born in the Lower Rhine Province of Germany, grew up as the only child of a cloth manufacturer and merchant. His family later relocated to the Netherlands, where he didn't display any notable academic prowess but developed a fondness for exploring the countryside. Unfortunately, Röntgen faced an unjust accusation of creating a caricature of one of his teachers, which led to his expulsion from technical school in Utrecht. He then pursued studies in mechanical engineering in Zurich, where he was able to enroll without the usual credentials of a regular student. Eventually, he obtained a PhD from the University of Zurich in 1869 and later became a professor of physics in 1876.
Natural Sources of Radiation
We are consistently subjected to measurable background radiation originating from various natural sources, with an average range of approximately 150–600 mrem per year (Figure 3). Among the contributors to background radiation are cosmic rays, which encompass high-energy particles and γ rays emitted by the sun and other stars. These cosmic rays continuously bombard the Earth. However, due to partial absorption by the atmosphere, individuals residing at sea level typically experience lower exposure (around 30 mrem per year) compared to those living at higher altitudes (approximately 50 mrem per year in Denver, Colorado). Additionally, each four-hour duration spent on an airplane at altitudes exceeding 30,000 feet results in an additional 1 mrem added to an individual's annual radiation exposure.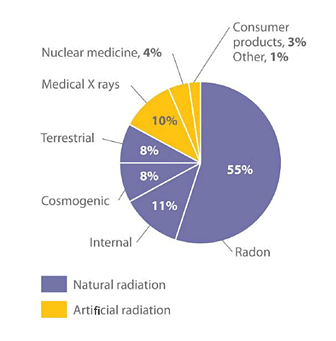 Figure 3: The Radiation Exposure of a Typical Adult in the United States: The average radiation dose from natural sources for an adult in the United States is about 150–600 mrem/yr. Radon accounts for more than half of an adult’s total radiation exposure, whereas background radiation (terrestrial and cosmogenic) and exposure from medical sources account for about 15% each. Data source: Office of Civilian Radioactive Waste Management.
Figure 3: The Radiation Exposure of a Typical Adult in the United States: The average radiation dose from natural sources for an adult in the United States is about 150–600 mrem/yr. Radon accounts for more than half of an adult’s total radiation exposure, whereas background radiation (terrestrial and cosmogenic) and exposure from medical sources account for about 15% each. Data source: Office of Civilian Radioactive Waste Management.
A second component of background radiation is cosmogenic radiation, produced by the interaction of cosmic rays with gases in the upper atmosphere. When high-energy cosmic rays collide with oxygen and nitrogen atoms, neutrons and protons are released. These, in turn, react with other atoms to produce radioactive isotopes, such as 14C: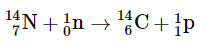 The carbon atoms react with oxygen atoms to form CO2, which is eventually washed to Earth’s surface in rain and taken up by plants. About 1 atom in 1 × 1012 of the carbon atoms in our bodies is radioactive 14C, which decays by beta emission. About 5000 14C nuclei disintegrate in your body during the 15 s or so that it takes you to read this paragraph. Tritium (3H) is also produced in the upper atmosphere and falls to Earth in precipitation. The total radiation dose attributable to 14C is estimated to be 1 mrem/yr, while that due to 3H is about 1000 times less.
The carbon atoms react with oxygen atoms to form CO2, which is eventually washed to Earth’s surface in rain and taken up by plants. About 1 atom in 1 × 1012 of the carbon atoms in our bodies is radioactive 14C, which decays by beta emission. About 5000 14C nuclei disintegrate in your body during the 15 s or so that it takes you to read this paragraph. Tritium (3H) is also produced in the upper atmosphere and falls to Earth in precipitation. The total radiation dose attributable to 14C is estimated to be 1 mrem/yr, while that due to 3H is about 1000 times less.
Terrestrial radiation forms the third major component of background radiation, arising from the remnants of radioactive elements that existed during Earth's early stages and their subsequent decay products. Various rocks and minerals present in the soil contain trace amounts of radioactive isotopes like 232Th, 238U, and their corresponding radioactive daughter isotopes such as 226Ra. The contribution of background radiation from these sources is roughly equivalent to that of cosmic rays, around 30 mrem per year. Building materials derived from rocks and minerals, such as brick or concrete-block houses, also contain these isotopes in small quantities, leading to significantly higher radiation exposure for individuals residing in such structures (ranging from 60 to 160 mrem per year) compared to those living in wooden houses (10-20 mrem per year). Our bodies also experience radiation absorption (approximately 40 mrem per year) from naturally occurring radioactive elements within us. As an illustration, the average adult possesses roughly 140 grams of potassium in the form of the K+ ion. Naturally occurring potassium includes 0.0117% of the isotope 40K, which undergoes decay by emitting both a β particle and a γ ray. Within the last 20 seconds, roughly the duration it took you to read this paragraph, approximately 40,000 40K nuclei disintegrated in your body.
By far the most important source of background radiation is radon, the heaviest of the noble gases (group 18). Radon-222 is produced during the decay of 238U, and other isotopes of radon are produced by the decay of other heavy elements. Even though radon is chemically inert, all its isotopes are radioactive. For example, 222Rn undergoes two successive alpha-decay events to give 214Pb: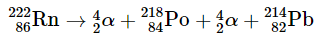 Radon, being a dense gas, has a tendency to accumulate in enclosed spaces like basements, especially in areas where the soil contains higher concentrations of naturally occurring uranium minerals. In most cases, the radioactive decay of radon is not a concern due to the very short range of the emitted α particle. However, if a radon atom happens to be present in the lungs at the time of decay, the chemically reactive daughter isotope polonium-218 can bind irreversibly to molecules within the lung tissue. Subsequent decay of 218Po results in the direct release of an α particle into one of the cells lining the lungs, leading to potential damage that can eventually result in lung cancer. Moreover, 218Po is readily absorbed by particles present in cigarette smoke, which adhere to the surface of the lungs and can trap the radioactive isotope. Recent estimates indicate that radon exposure contributes to approximately 15% of lung cancer deaths. Recognizing the potential health risks associated with radon, many states have implemented requirements for radon testing before houses can be sold. According to current estimates, radon is responsible for over half of the radiation exposure experienced by the average adult in the United States.
Radon, being a dense gas, has a tendency to accumulate in enclosed spaces like basements, especially in areas where the soil contains higher concentrations of naturally occurring uranium minerals. In most cases, the radioactive decay of radon is not a concern due to the very short range of the emitted α particle. However, if a radon atom happens to be present in the lungs at the time of decay, the chemically reactive daughter isotope polonium-218 can bind irreversibly to molecules within the lung tissue. Subsequent decay of 218Po results in the direct release of an α particle into one of the cells lining the lungs, leading to potential damage that can eventually result in lung cancer. Moreover, 218Po is readily absorbed by particles present in cigarette smoke, which adhere to the surface of the lungs and can trap the radioactive isotope. Recent estimates indicate that radon exposure contributes to approximately 15% of lung cancer deaths. Recognizing the potential health risks associated with radon, many states have implemented requirements for radon testing before houses can be sold. According to current estimates, radon is responsible for over half of the radiation exposure experienced by the average adult in the United States.
Artificial Sources of Radiation
Apart from natural background radiation, humans are also exposed to small doses of radiation from various artificial sources. One significant source is the use of x-rays for diagnostic purposes in medical and dental settings. These x-rays, which have lower energy compared to γ rays, contribute to radiation exposure. For instance, a single chest x-ray delivers a radiation dose of approximately 10 mrem, while a dental x-ray provides about 2-3 mrem. Other minor sources of radiation include cathode-ray tubes found in television screens and computer monitors, which emit x-rays. Previously, radium was used in luminescent paints for watch dials, but its highly toxic nature led to its replacement with tritium (3H) and promethium (147Pr). These substances emit low-energy β particles that are absorbed by the watch crystal or the glass covering the instrument. The combined radiation exposure from television screens, monitors, and luminescent dials amounts to approximately 2 mrem per year. Residual fallout from past atmospheric nuclear-weapons testing is estimated to contribute around twice this amount, while the nuclear power industry's impact is less than 1 mrem per year (equivalent to a single 4-hour jet flight).
Example 1: Calculate the annual radiation dose in rads a typical 70 kg chemistry student receives from the naturally occurring 40K in his or her body, which contains about 140 g of potassium (as the K+ ion). The natural abundance of 40K is 0.0117%. Each 1.00 mol of 40K undergoes 1.05 × 107 decays/s, and each decay event is accompanied by the emission of a 1.32 MeV β particle.
Given: mass of student, mass of isotope, natural abundance, rate of decay, and energy of particle
Asked for: annual radiation dose in rads
Strategy:
A. Calculate the number of moles of 40K present using its mass, molar mass, and natural abundance.
B. Determine the number of decays per year for this amount of 40K.
C. Multiply the number of decays per year by the energy associated with each decay event. To obtain the annual radiation dose, use the mass of the student to convert this value to rads.
A. The number of moles of 40K present in the body is the total number of potassium atoms times the natural abundance of potassium atoms present as 40K divided by the atomic mass of 40K:
B. We are given the number of atoms of 40K that decay per second in 1.00 mol of 40K, so the number of decays per year is as follows:
C. The total energy the body receives per year from the decay of 40K is equal to the total number of decays per year multiplied by the energy associated with each decay event:
We use the definition of the rad (1 rad = 10−2 J/kg of tissue) to convert this figure to a radiation dose in rads. If we assume the dose is equally distributed throughout the body, then the radiation dose per year is as follows:
This corresponds to almost half of the normal background radiation most people experience.
Example 2: Because strontium is chemically similar to calcium, small amounts of the Sr2+ ion are taken up by the body and deposited in calcium-rich tissues such as bone, using the same mechanism that is responsible for the absorption of Ca2+. Consequently, the radioactive strontium (90Sr) found in fission waste and released by atmospheric nuclear-weapons testing is a major health concern. A normal 70 kg human body has about 280 mg of strontium, and each mole of 90Sr undergoes 4.55 × 1014 decays/s by the emission of a 0.546 MeV β particle. What would be the annual radiation dose in rads for a 70 kg person if 0.10% of the strontium ingested were 90Sr?
5.7 × 103 rad/yr (which is 10 times the fatal dose)
Assessing the Impact of Radiation Exposure
The potential risk to human health posed by the combined radiation exposure from both artificial and natural sources is currently a contentious topic in public policy debates. Table 2 provides information on the effects of radiation from individual doses of varying magnitudes on humans. Due to numerous factors influencing radiation exposure, such as exposure duration, source intensity, particle energy, and type, it is challenging to precisely quantify the specific hazards associated with one radioisotope compared to another. Nevertheless, there are generally accepted conclusions regarding the effects of radiation exposure that are considered valid.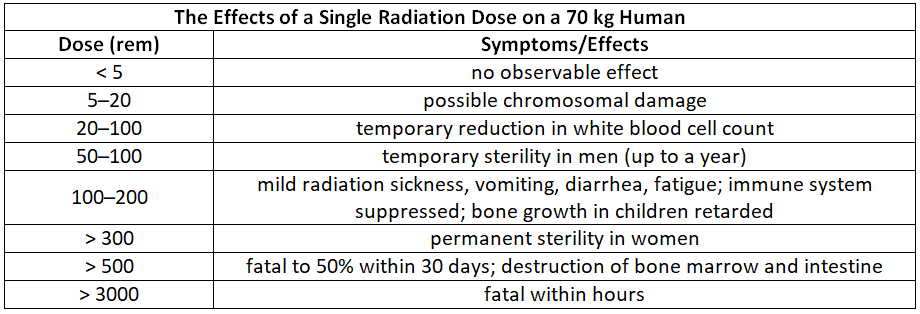
Exposure to radiation doses of 600 rem or higher is consistently lethal, and a dose of 500 rem results in a 50% fatality rate within 30 days. Smaller doses, up to 50 rem, seem to have limited health effects despite being equivalent to several decades of natural radiation exposure. However, this does not imply that such doses have no negative consequences; they can potentially lead to long-term health issues like cancer or genetic alterations that affect future generations. Assessing the potential harmful effects of much smaller doses from artificial sources (< 100 mrem per year) is more challenging.
The tissues that are most affected by high levels of radiation exposure throughout the entire body include bone marrow, intestinal tissue, hair follicles, and reproductive organs, all of which consist of rapidly dividing cells. This susceptibility of rapidly dividing cells to radiation exposure is why radiation is often used in cancer treatment. Cancer cells, which divide more rapidly than normal cells, are preferentially destroyed by radiation.
Studies conducted on fruit flies, examining the long-term effects of radiation exposure, have shown a linear relationship between the number of genetic defects and both the dose magnitude and the duration of exposure. In contrast, similar studies conducted on mice indicate a significantly lower number of defects when a given radiation dose is spread out over an extended period rather than being received all at once. Both patterns are depicted in Figure 4, raising the question of which model applies to humans.
According to one hypothesis, mice have a low risk of harm from low doses of radiation because their bodies have mechanisms to deal with the damage caused by natural radiation. However, at much higher doses, their natural repair mechanisms become overwhelmed, resulting in irreversible damage. Since mice share greater biochemical similarities with humans than fruit flies do, many scientists believe that this model is also applicable to humans. On the other hand, the linear model assumes that all radiation exposure is inherently damaging and advocates for stringent regulation of low-level radiation exposure. The accuracy of each viewpoint remains unknown, but determining the answer holds significant implications for regulating radiation exposure.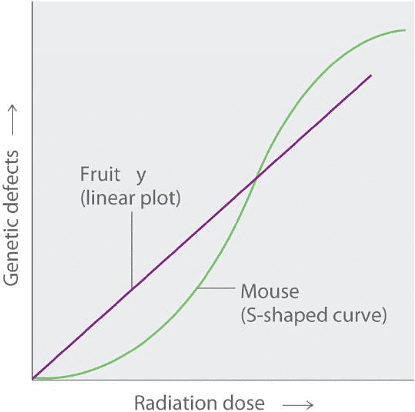
Figure 4: Two Possible Relationships between the Number of Genetic Defects and Radiation Exposure: Studies on fruit flies show a linear relationship between the number of genetic defects and the magnitude of the radiation dose and exposure time, which is consistent with a cumulative effect of radiation. In contrast, studies on mice show an S-shaped curve, which suggests that the number of defects is lower when radiation exposure occurs over a longer time. Which of these relationships is more applicable to humans is a matter of considerable debate.
Graph of genetic defects against radiation dose. Fruit is graphed in purple and has a linear plot. Mouse is graphed in green and has a S-shaped curve.
Summary
- Nonionizing radiation possesses relatively low energy and can function as a source of heat, while ionizing radiation, with higher energy levels, has the ability to penetrate biological tissues and exhibits high reactivity. The impact of radiation on matter is contingent upon its energy level. Nonionizing radiation, characterized by relatively low energy, transfers its energy to matter primarily in the form of heat. On the other hand, ionizing radiation, possessing higher energy levels, can remove an electron from an atom upon collision, resulting in the formation of a positively charged ion that can cause damage to biological tissues. Alpha particles have limited penetration capabilities, whereas γ rays can penetrate deeper into matter.
- The measurement units commonly employed to quantify radiation exposure, or dose, include the roentgen (R), which quantifies the energy absorbed by dry air, and the rad (radiation absorbed dose), which measures the radiation required to generate 0.01 J of energy within 1 kg of matter. The rem (roentgen equivalent in man) is utilized to assess the actual tissue damage caused by a specific amount of radiation.
- Natural sources of radiation encompass cosmic radiation, which comprises high-energy particles and γ rays emitted by the sun and other stars; cosmogenic radiation, generated by the interaction of cosmic rays with gases in the upper atmosphere; and terrestrial radiation originating from radioactive elements present on Earth since its inception, as well as their subsequent decay products.
- The risks associated with ionizing radiation depend on factors such as the intensity of the radiation, the manner of exposure, and the duration of the exposure.
|
110 videos|130 docs|117 tests
|