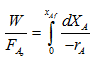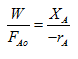Catalysts Test and Reactors Types - Chemical Engineering PDF Download
Reactor types and catalysts test
Reactor types
Reactors can be classified based on different criterion such as size, reactor material, methods of charging and discharging or type of fluid flow. For fluid – solid heterogeneous system reaction rate is based on mass of solid catalyst rather than on reactor volume. In laboratory scale, the reactions are carried out in micro reactors with diameters ranging from 1 – 5 mm for testing up to 0.1 – 1gm of catalysts. In industry, diameter and height of reactors can vary from 1 to 10 m and 2 to30 m respectively. The reactor materials also vary depending on usage. Laboratory reactors are usually made of glass, quartz or stainless steel with few mm wall thicknesses. The large industrial reactors depending on usage are usually made of mild steel or stainless steel or other alloys. The wall thickness can range from of 6-15 mm depending on process pressure. Depending on the operation, reactor can be batch, mixed flow or packed bed type. The choice of reactor depends on the type of reaction. For examples, many hydrogenation reactions are carried out in CSTR while many of the solid catalysts are tested in packed bed reactor. Reactor selection also depends on the type of catalyst and its activity, selectivity and deactivation behavior. The schematic diagram of different type of reactors is shown in Fig 1.
For study of catalytic reactions in laboratory different reactors are used. For example fluid-solid catalytic reactions are studied in (1) packed bed reactors heated in furnace, (2) Carberry reactors equipped with rotating catalyst basket and cooling or heating jacket or (3) Berty reactor with internal circulation and cooling or heating jacket. For reactions involving gas-liquid-solid, slurry CSTR with cooling or heating jacket or packed bed reactor with downflow, upflow or countercurrent flow of gas and liquids are used. Laboratory reactors are mainly used for measuring reaction kinetics and catalyst activity at different conditions of temperature and pressure.
Fixed bed reactor
The fixed bed or packed bed reactors are most commonly used for study of solid catalyst. A fixed bed reactor usually consists of a cylindrical vessel packed with catalyst pellets and easy to design and operate. The metal support grid and screen is placed near the bottom to support the catalyst. Inert ceramic balls are placed above the catalyst bed to distribute the feed evenly.
Advantages of packed bed or fixed bed reactor include ideal plug flow behavior, lower maintenance cost and reduced loss due to attrition and wear. Heat management is very important aspect for design of fixed bed reactor. Poor heat distribution may result in non uniform reaction rates and consequently low reactant conversion. Poor heat transfer may also result in generation of hot spots and thermal degradation of catalyst. However, the situations are observed more in large fixed bed and for highly exothermic or endothermic reactions when temperature control is difficult. The regeneration or replacement of catalyst is also difficult in fixed bed reactors and process needs to be shutdown. Another major disadvantage of packed bed reactor is plugging of bed due to coke deposition which results in high pressure drop. High pressure drop is also observed for small beads or pellets of catalysts. However, increase in pellet size increases the pore diffusion limitation.
Catalyst pellet sizes are usually in the range of 1 to 10 mm. Non-uniform packing of catalysts can cause channeling of fluids leading to poor heat and mass transfer. The column to particle diameter is maintained in between 10 to 20 to minimize channeling. The bed voidage is usually 70 to 90 %. Plug flow behavior is ensured by maintaining ratio of reactor length to catalyst particle diameter greater than 50. The allowed pressure drop is less than 0.5 inch water per foot of bed depth. Usually the ratio of bed height to diameter is maintained greater than 0.5.
For better heat management for very highly exothermic (or endothermic) reaction the multitubular reactor is used with catalyst packed inside the tubes. The cooling (or heating) fluid flows through the shell side. The length is limited by allowable pressure drop. The multitubular reactor has high surface area for heat transfer per unit volume. For determination of heat transfer and mass transfer properties several correlations are available in literature.
Fluidized bed reactors
In fluidized bed reactor catalyst pellets of average size less than 0.1 mm are fluidized by the reactant fluid. The linear velocity is maintained above the minimum fluidization velocity required to obtain the fluidized bed. As the superficial velocity increases, the bed expands and become increasingly dilute. At high enough linear velocity, the smallest catalyst particles escape from the bed and have to be separated from exhaust gases and recycled.
In fluidized bed, heat transfer is much better resulting in more uniform temperature compared to packed bed reactor. Frequent regeneration of catalyst can be done without any shutdown of the process. However, fluidized bed is a complicated system to operate and requires extensive investments and high operating and maintenance cost. Other major disadvantages are attrition and loss of catalysts due to fluidized condition. Modeling of fluidized bed flow is complex. The fluidized bed is assumed to consist of bubble and emulsion phases which can be modeled respectively by plug flow and CSTR, as the emulsion phase is assumed to be well mixed. Correlation for heat and mass transports are available in literature. The reactor is extensively used for catalytic cracking process.
Carberry reactor or Berty reactor
For catalytic investigations, reactors equipped with rotating basket or fixed basket with internal circulation can be used. These CSTR type reactors are used to minimize the inherent mass and heat transfer limitations observed in fixed-bed reactors. These reactors are frequently used in industry to evaluate reaction mechanism and reaction kinetics. The most common type of reactors used are Carberry and the Berty reactors.
The main feature of the Carberry reactor is that the catalyst particles are contained in a spinning basket or embedded in the blades of a spinning agitator. The mounted catalyst is rapidly rotated resulting in good mixing between reactants in fluid phase and the solid catalyst. This minimizes the mass transfer and heat transfer resistances. The basket or impellers can spin up to 2,500 rpm.
The Berty reactor uses an internal recycling to achieve perfectly mixed behavior. The catalyst is contained in a fixed bed basket through which the reacting gases circulate. The catalyst basket is equipped with large diameter impellers rotated in order to circulate gases & liquids past solid catalysts. An internal recirculation rate of 10 to 15 times of the feed rate effectively eliminates external diffusion resistance and temperature gradient. Retaining the solid catalyst in a spinning woven wire mesh basket allows gas / liquid circulation with low pressure drop. Circulating the reactants past the catalyst minimize wearing & breakage.
Multiphase reactors
Slurry reactors
The catalytic reaction can also be carried out in two–phase or three –phase stirred tank reactors also known as slurry reactors. In three –phase reactor, gas and liquid reactants are brought into contact with solid catalyst particles. In two–phase reactor, fluid phase is usually liquid reactant in contact with the solid catalyst. The reaction of gaseous reactant with catalyst is usually carried out in fixed bed reactor. In three –phase slurry reactor the gaseous reactant and solid catalysts are dispersed in continuous liquid phase by mechanical agitation using stirrer. The efficient stirring ensures nearly uniform composition throughout the reactor. This kind of reactor is used in hydrogenation, oxidation, halogenations and fermentation process. The advantages include nearly isothermal operation and good heat and mass transfers. The use of powder catalysts having high activity minimizes the intraparticle diffusion limitation. The reactors can be operated in batch, semi batch or continuous mode. In three – phase system bubbles of gas rise through agitated slurry. Solid particles are in size range of 0.01to 1.0 mm. The solid concentration can be up to 30 vol. %. Lower concentration is also used. In hydrogenation of oil with nickel catalyst, the solid content is 0.5 vol. %. The external transport effects are important in slurry reactors and details are discussed in lecture no. 30. Hydrogenation of oils is carried out in slurry of nickel catalyst particles. Industrial hydrogenation reactors are usually of the size in the range of 500-200 L. The reactors are operated up to pressure of 200 atm and temperature of 350°C. The reactors are equipped with internal agitator, gas inlet, facility for insitu sampling and heater or cooler for temperature control.
Trickled bed reactors
In trickled bed reactor gaseous and liquid reactants flow co-currently downward over a packed bed of solid catalyst particles. The liquid is distributed across the reactor cross section by a distributor plate. The gas enters at the top and distributed along with the liquid. The liquid flows downward by gravity and drag of the gas. For low liquid flow rates and low to moderate gas flow rates, the gas phase is continuous with liquid trickling down forming film over the solid catalyst. The thickness of the liquid film has been estimated to vary between 0.01 and 0.2 mm. This flow regime is known as a trickle flow regime. The fluid approaches plug flow leading to higher conversion than slurry reactors for the same reactor volume. Other advantages include ease of installation, minimal catalyst handling and low catalyst attrition as in packed bed reactor. Disadvantages include maldistribution of flow resulting in channeling or bypassing, possibility of non uniformity in packing, incomplete contacting or wetting and intraparticle diffusion resistance. Catalyst bed depth is limited by pressure drop, catalyst crush strength and maximum adiabatic temperature increase for stable operation. The reactor length to diameter ratio can vary between 1 and 10 depending on the allowable pressure drop. Other parameters important for trickled bed include void fraction of bed, holdup for phases, wetting efficiency (fraction of catalyst wetted by liquid), gas – liquid mass transfer coefficient, liquid–solid mass transfer coefficient, liquid and gas mixing, pressure drop and heat transfer coefficients. The wetting efficiency of the catalyst is important for reaction rate and increases with increasing liquid rate. The trickle bed reactor is most commonly used for hydrogenation and hydrodesulfurization reactions.
Bioreactors
In bioreactor live cells or enzymes are used as catalyst to perform the biochemical reactions. Bioreactor operations are limited by the conditions favorable for the biological systems. Most living cells can tolerate only mild conditions of temperature and pH. Hence in bioreactors stringent control of temperature, pH or any contamination is needed. Bioreactor may have two phases, liquid-solid as in anaerobic process or three phases, gas, liquid and solid as in aerobic process. The solid phase typically contains the cells (bacteria, fungi, algae etc.) that serve as biocatalyst. The density of biocatalytic phase is close to water. The biocatalyst can also be used in immobilized form in which cells are trapped within solid or semi solid structure such as porous particles or gel. Liquid is primarily water with dissolves the feed and products. In aerobic bioreactor the gas phase consists of primarily air and product gas CO2. Bioreactors are mainly operated in batch or semi batch mode allowing better control of process parameters. Increasing number of bioreactor is operated in continuous mode such as in wastewater treatment, lactic acid production, production of human insulin etc.
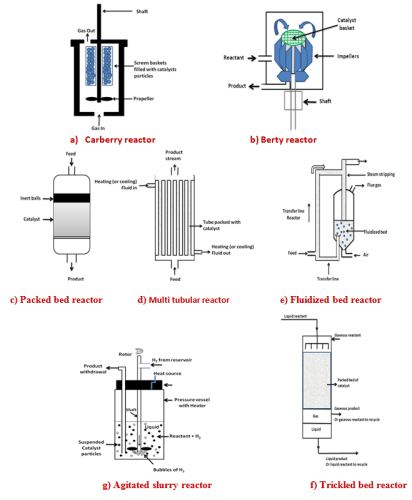
Fig. 1. Schematic diagram of different type of reactors
Catalyst tests
Activity and selectivity
The performance of catalyst is characterized by its activity for reaction and selectivity for a product. The activity of a catalyst for a reaction at specified conditions is generally expressed in terms of rate of reaction at that condition over the catalyst. The catalyst that shows higher rate of reaction at the given conditions is said to have higher activity. For solid catalyzed reaction, rate can be defined as, r = g mol of reacted /s. g of catalyst. Here mass of solid is used because amount of catalyst is important. The reactor volume that contains the catalysts is of secondary importance. Activity is also expressed in terms of conversion of a reactant achieved. Higher the conversion at given conditions, higher is the activity of the catalyst for that reaction. The conversion can be defined as follows

For supported metal catalysts, defining activity in terms of metal sites is more useful as the metal sites actually act as the active sites for chemical reactions. Hence, activity is often expressed in terms of turnover frequency or TOF. TOF is defined as the number of molecules reacting per active site per second (s-1). Though the TOF value is expected to be constant for a particular metal catalyst for a given reaction at specified conditions, however, in reality significant difference may be observed due to differences in catalyst preparation, metal support interaction, crystallite size, surface structure and morphology etc.

The next important parameter determining the performance of catalyst is selectivity for a particular product. The selectivity of a product X can be defined in several ways. One of the definitions is shown below:
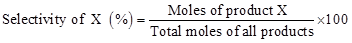
The performance of catalyst is tested in a suitable experimental setup. Fig. 2 shows a schematic diagram of a simple experimental setup for studying gas phase reaction over solid catalysts. Typically, solid catalysts are tested in a tubular down-flow reactor. A simple manometer or any pressure gauge can be included before the reactor to ensure that the pressure drop is within acceptable limits. All the reactants are combined in a mixer and sent to the reactor where it comes in contact with the catalyst bed. The product gases or liquids are usually analyzed using a gas chromatograph equipped with suitable columns. The columns are selected so that the product compounds are separated efficiently giving distinct peaks that can be easily identified and quantified. Further detector type and temperature as well as carrier gas type and flow rates are also important parameters determining the retention time and peak area in a chromatograph. Catalytic tests are carried out at predetermined range of temperature, pressure, feed composition and total flow rates depending on reactions. The catalysts amount range to be used is also fixed according to the requirement.
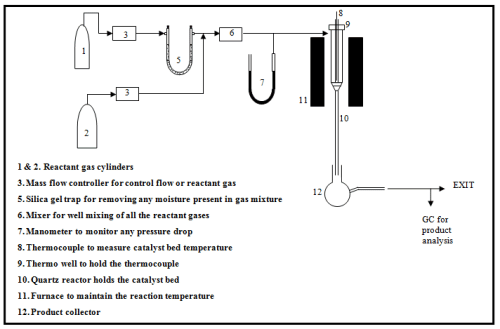
Fig. 2. Schematic diagram of a typical experimental setup
2.3.2.1. Collecting data from laboratory reactors
Kinetic data are collected for the following purposes:
• Determination of activity-selectivity and deactivation data for catalyst selection
• Determination of reaction mechanism and kinetic parameters for understanding the reaction at the fundamental level so the reaction process can be modeled which can be used for design of reactors.
Investigation of the reaction kinetics is done at steady state conditions for most active and selective catalyst. The effect of temperature and partial pressures of reactants on the activity and selectivity is investigated in the desired temperature range. The process of data collection typically involve three major steps:
- Selection of a reaction and catalyst
- Selection of reactor type
- Analysis of data
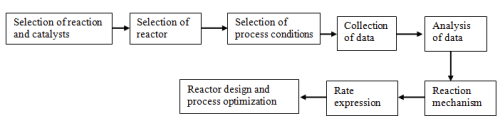
Fig. 2. Steps for obtaining and analyzing kinetic data
To measure the specific catalytic activity or intrinsic reaction kinetics, the data must be collected in absence of any pore diffusion limitations, mass transfer limitations or heat transfer limitations. For analysis of data collected in the presence of mass transfer or heat transfer limitations, suitable criterion should be included during analysis. Deactivation of catalysts should be also avoided during data collection. These will be discussed in detail in later sections. Further data should be collected over a wide range of temperature and reactant concentrations to provide adequate reaction model that can be applicable at wide temperature and concentration range.
Mode of reactor operations
Reactors can be operated in two ways:
- Integral reactors
- Differential reactors
Integral reactors
When a reactor is operated at high conversion, the reactor is said to be an integral reactor. When reaction takes place in a packed bed reactor at high conversion there is possibility of wide variation of conversion and reaction rate within the reactor even under isothermal conditions. Hence, the integral form of design equation is to be used. This is the basis for design for an ideal packed bed reactor.
| (1) |
Differential reactors
When the reaction in a tubular reactor is carried out at very low conversions the changes in conversion and reaction rate across the reactor are small enough to be neglected and the rate can be considered constant across the reactor. Then the rate in above equation [1] can be considered constant and taken out of the integral and the equation can be simplified to
|
This above equation is same as for mixed flow reactor or CSTR. In this case analysis of data is simplified.
FAQs on Catalysts Test and Reactors Types - Chemical Engineering
| 1. What is a catalyst and how does it work in chemical reactions? |  |
| 2. What are the different types of catalysts used in chemical engineering? |  |
| 3. What are the main factors that affect the performance of a catalyst in a chemical reaction? |  |
| 4. What are the advantages of using catalysts in chemical reactions? |  |
| 5. What are the different types of reactors used in chemical engineering? |  |