Catalysts Drying, Calcination & Formulations - Chemical Engineering PDF Download
Washing and filtering
Washing can be done by decantation. This method is time consuming. In this method the precipitate or gel is added to a large volume of distilled water and the suspension is thoroughly stirred. Then, the suspension is allowed to settle. The foreign undesirable ions are desorbed from particles as they settle down slowly at the bottom. When a clear interface is visible, the water is removed by decantation and the process is repeated. The number of washings required is determined by checking the impurity level of the decanted water. After washing, the precipitate or gel is filtered. The process can be reversed. That is the filtration is done first and the precipitate or gel is washed with distilled water in the subsequent step. This method takes less time. Impurity level in the wash water is checked to determine the required number of washings.
Drying
Drying is described as the elimination of water or solvent from the pores of the precipitate or gel. It can be done in two ways:
- Solvent evaporation
- Super critical drying
Solvent evaporation : This type of drying is done in a conventional oven at 100-200 °C and is generally accompanied by a contraction of the structure. In case of gel the product obtained from ordinary drying is known as dry gel or xero gel. Initially drying occurs through evaporation of moisture from the outside surface of the materials. The rate of water loss is constant and the mass transfer is controlled by temperature, relative humidity and flow rate of air over the surface and the size of the filtrate. The process continues until the moisture content drops to about 50%. Continued moisture loss occurs with a declining rate in which evaporation is controlled by capillary forces. The saturation point decreases as the pore become smaller and the evaporation slows until water is forced into larger pores by concentration gradient. At the moment of drying, as the pore liquid is evaporated from a gel network, the capillary pressure associated with the liquid vapor interface within a pore can become very large for small pores. The capillary pressure that can develop in a pore of radius r is 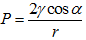 , where P is capillary pressure, γ is surface tension, rthe pore radius and α is the contact angle between liquid and solid. The capillary pressure with water evaporating from a pore with a radius of 1 nm is in the order of 1480 atm. Large capillary tension can lead to collapse of internal structure resulting in loss of pore volume and surface area. This phenomenon is more significant for a gel having more intricate porous structure compared to ordinary precipitated material. For a given pore size the capillary pressure can be reduced by
, where P is capillary pressure, γ is surface tension, rthe pore radius and α is the contact angle between liquid and solid. The capillary pressure with water evaporating from a pore with a radius of 1 nm is in the order of 1480 atm. Large capillary tension can lead to collapse of internal structure resulting in loss of pore volume and surface area. This phenomenon is more significant for a gel having more intricate porous structure compared to ordinary precipitated material. For a given pore size the capillary pressure can be reduced by
- Using a solvent with a lower surface tension or with a contact angle close to 90°.
- Eliminating the liquid vapor interface altogether with either super critical or freeze drying.
If temperature gradient is high so that evaporation rate is much faster compared to removal of moisture that is slowed by smaller pores, then large internal pressure of steam develops and also leads to collapse of structure. Therefore, high temperature gradient in the sample must be avoided. Drying at a lower temperature gives less surface area loss since evaporation rates are lower.
Supercritical drying is aimed at eliminating the liquid vapor interface and the accompanying capillary pressure responsible for structure collapse during conventional drying particularly for gels. It is used where retention of original micro structure of the product is important. This process typically involves displacement of water using an alcohol followed by removal of this alcohol/water mixture using supercritical carbon dioxide. In this process the gels are placed in an autoclave filled with ethanol. The system is pressurized to at least 750-850 psi with CO2 and cooled to 5-10°C. Liquid CO2 is then flushed through the vessel until all the ethanol has been removed from the vessel and gels. When the gels are ethanol-free the vessel is heated to a temperature above the critical temperature of CO2 (31°C). As the vessel is heated the pressure of the system rises. CO2 is carefully released to maintain a pressure slightly above the critical pressure of CO2 (1050 psi). The system is held at these conditions for a short time, followed by a slow, controlled release of CO2 to ambient pressure. As with previous steps, the length of time required for this process is dependent on the thickness of the gels. The process may last anywhere from 12 hours to 6 days.
Calcinations or sintering
After the removal of pore liquid, further heat treatment is necessary to convert the precipitate or dry gel to catalytically useful form. After drying, the next step of heat treatment is known as calcination. Often the heating is done in the presence of flowing air or oxygen to burn any residual organics or to oxidize the sample. Multiple changes occur during this process including:
- Active phase generation: The hydroxide form is converted to oxide form.
- Stabilization of mechanical properties: The catalysts sample is subjected to a more severe heating treatment than that is likely to encounter in a reactor to ensure the stability of its textural and structural properties during reaction.
- Loss of chemically bound water: The chemically bound water is removed at higher temperature.
- Changes in pore size distribution and surface area due to sintering: Exposing the sample to high temperature over an extended period of time leads to some sintering and consequently decrease in surface area.
- Change in phase distribution: Higher temperature cause material to crystallize into different structural forms. Fig. 1 shows the formation of various phases of alumina when calcined at different temperatures.
The extent of change in the physical characteristics of the final sample depends on following parameters: temperature, heating rate, heating time and gaseous environment.


Fig. 1. Formation of various phases of alumina on calcination at different temperatures
Catalyst shaping and formulation
Mainly, solid catalysts are used in industrial catalytic processes and these are formulated in different forms such as pellets, extrudates, granules or sphere form.
Formulation and shaping of solid catalysts is done to:
a. avoid high pressure drop in fixed and moving beds
b.increase thermal resistance against sintering fracture or phase transition
c.increase mechanical resistance against crushing and attrition
d.ensuring high effective heat conductivity in fixed and moving bed for strongly exothermic and endothermic reactions
Aiming at highest catalyst efficiency is the primary objective in catalyst design because conversion, selectivity and thermal resistance are strongly affected by above mentioned parameters. Some of the common catalysts formulation techniques are :
- pellet formation
- granulation
- extrusion
- spray drying
Pellet formation:
It is a high pressure agglomeration technique producing particles of uniform shape and dimensions. Typically the dry catalyst powders are compressed in a dye by applying forces between 50-80 kN with a pending tool. Factors such as ultimate tensile strength of the materials , moisture content, porosity, stickiness are important. Some materials, such as kieselguhr, undergo easy pellet formation whereas other materials such as alumina require addition of small amount of plasticizers or lubricants such as graphite, talc etc. Important processing parameters are the maximum applied pressure and the rate of pressure rise. Both influence the hardness of the pellets as well as the integrity of compacted particles.
Granulation:
This is a size enlargement process by wet tumbling. In this method the particles are tumbled in a cylinder A cohesive liquid is sprayed onto the catalyst powder such that the wetted particles stick together. The granules grow by contacting further particles. Product with wide size distribution can be produced by controlling parameters such as binders type and concentration, rpm of pan, granulation time and angle of inclination of pan. Typically pan granulation yields spherical particles of diameter in the range of 2-20 mm.
Extrusion:
It is a widely used technique. In this method, a suspension or paste of the catalyst powder is passed through a profiled die that determines the shape of the body. Screw extruders are very common in use. Slurry of the catalyst is fed to the extrudate at one end and the screw forces the slurry through the holes at the other end. As the ribbon of slurry emerges from the holes, a knife is arranged at the end to cut it to the required size. Particles of narrow size distribution can be obtained by this method.
Spray drying:
This process involves atomization of slurry feedstock into a spray of droplets and contacting the droplets with hot air in a drying chamber. Particle sizes are determined by the size of droplets, which is controlled by design of spray nozzles, slurry flow rate, slurry viscosities. Products in a spray dryer are spheres of diameters in the range of 0.05 to 0.5 mm.
The schematic diagram of catalyst formulation techniques are shown in Fig. 2.

Fig. 2. Schematic diagram of different catalysts formulation techniques
FAQs on Catalysts Drying, Calcination & Formulations - Chemical Engineering
| 1. What is the purpose of catalyst drying? |  |
| 2. What is the difference between catalyst drying and catalyst calcination? |  |
| 3. What are some common methods used for catalyst drying? |  |
| 4. Why is calcination important in catalyst preparation? |  |
| 5. Can catalyst formulations be customized for specific reactions? |  |

















