Sewage & Sullage Treatment | Civil Engineering SSC JE (Technical) - Civil Engineering (CE) PDF Download
| Table of contents |

|
| Sewage and Sullage Treatment, Quantity and Characteristics of Waste Water |

|
| Sullage |

|
| Sewage |

|
| Aerobic Decomposition |

|
| Anaerobic Decomposition |

|
| Biochemical Oxygen Demand (BOD) |

|
Sewage and Sullage Treatment, Quantity and Characteristics of Waste Water
Sullage
This refers to the wastewater generated from bathrooms, kitchens, washing place and wash basins etc.
The composition of this waste does not involve a higher concentration of organic matter and is less polluted water than sewerage.
Sewage
It indicates the liquid waste originating from the domestic uses of water. It includes Sullage, discharge from toilets, and urinals, wastewater generated from commercial establishments, institutions, industrial establishments, and also the groundwater, and stormwater that may enter into the sewers. Its decomposition produces large quantities of malodorous gases and it contains numerous pathogenic bacteria, along with the concentration of organic matter and suspended solids.- Domestic sewage: Consists of liquid wastes originating from urinals, bathrooms, kitchen sinks, wash basins etc. This sewage is generally extremely foul, because of the presence of human excreta.
- Industrial sewage: Liquid wastes originating from industrial processes of various industries like dying, paper making, brewing etc.
Aerobic Decomposition
If air or oxygen is available freely to waste water in dissolved form, then the biodegradable organic matter will undergo aerobic decomposition, caused by aerobic bacteria as well as by facultative bacteria. This bacteria will then utilizes the free oxygen as electron acceptor, thereby oxidizing the organic matter to stable and unobjectionable end products. Stable end products like nitrates, carbon dioxide, and sulfates are formed.
-Nitrogenous organic matter on oxidation gives NO3- + NH3 + Energy
-Carbonaceous organic matter on oxidation gives CO2 + H2O + Energy
-Sulphurous organic matter on oxidation gives SO4-- + Energy
Anaerobic Decomposition
If free dissolved oxygen is not available in the sewage, then anaerobic decomposition, called putrefaction will occur. Anaerobic bacteria as well as facultative bacteria operating anaerobically will then flourish and convert the complex organic matter into simpler organic compounds of nitrogen, carbon, and sulphur.
Nitrogenous organic matter on reduction gives N2 + NH3 + Organics Acids + Energy
Carbonaceous organic matter on reduction gives CO2 + Energy
Sulphurous organic matter on reduction gives H2S + Energy
Aerobic bacteria: Flourish in the presence of free dissolved oxygen in wastewater and consume organic matter for their food thereby oxidizing it to stable end products.
Anaerobic bacteria: Flourish in the absence of oxygen
Facultative bacteria: Can operate either as aerobically or as anaerobically.
Characteristics of Sewage
1. Physical characteristics:
- Turbidity: Sewage is normally turbid, resembling dirty dishwater. It is measured by a turbidity rod or turbidity meter.
- Colour: If the colour is yellowish,grey or light brown,it indicates fresh sewage. However, if the colour is black or dark brown,it indicates stale sewage.
- Odour: Measured as Threshold odor number (TON), which represents the extent of dilution required to just make the sample free of odor.
- Temperature: It has an effect on the biological activity of bacteria present in sewage and it also affects the solubility of gases in sewage. The normal temperature of the sewage is generally higher than the temperature of water, because of additional heat added during the utilisation of water.
2. Chemical characteristics:
- Total solids, suspended solids, Settleable solids, dissolved solids.
Total solids: Determined by evaporating a known volume of sewage sample and weighing the dry residue left. The mass of the residue divided by the volume of the sample evaporated will represent the total solids in mg/L.
Suspended solids: Those solids which are retained by a filter of 1 um pores.
Dissolved solids: Total - suspended.
Total Suspended solids= Volatile solids + Fixed solids.
Loss of weight due to burning of suspended solids is called volatile solids and residue is called fixed solids. - Settleable solids: This can be determined by the Imhoff cone. The capacity of the cone is 1 litre and it is graduated up to about 50ml. Sewage is allowed to stand in this cone for a period of 2 hours and the quantity of solids settled at the bottom of the cone can then directly be read out.
- Ph value: It is an indicator of the alkalinity of the sewage. The lesser is the Ph value, the lesser is the alkalinity. Its determination is important because of the fact that the efficiency of certain treatment methods depends upon the availability of a suitable Ph value.
- Chloride content: The Normal chloride content of sewage is 120. Higher values indicate the mixing of industrial wastes.
The chloride content can be measured by titrating the wastewater with standard silver nitrate solution, using potassium chromate as an indicator. - Nitrogen content: The presence of Nitrogen in sewage indicates the presence of organic matter and may occur in one or more of the following forms:
1. Free ammonia
2. Organic nitrogen or Albuminoid nitrogen
3. Nitrites
4. Nitrates.
The sum total of free ammonia and organic nitrogen is called as Kjeldahl nitrogen. Free ammonia indicates the quantity of nitrogen present in sewage before the decomposition of organic matter is started. The nitrites indicate the presence of partly decomposed organic matter. Nitrates indicate the presence of fully oxidized organic matter. In drinking water nitrates should be less than 45mg/ . Excess of nitrates causes blue baby or methemoglobinaemia disease in children. - Presence of fats, oils, and greases: It interferes with the treatment process like filtration so needs to be removed.
- Sulphides, sulphates & H2S gas: Cause smell and corrosion of concrete sewer lines
- Dissolved oxygen: (DO): The DO content of sewage is determined by Winkler’s method. While discharging into streams, DO in sewage must be more than 4 ppm to protect fish. If the temperature of the sewage is higher, the DO content will be less.
- Chemical oxygen demand: Oxygen is required to completely oxidize the organic matter to CO2, H2O and other oxidized species.
- Total organic carbon: Another important method of expressing organic matter is in terms of its carbon content. Known concentrations of chemical components in wastewater will enable us to calculate the carbon content present in sewage.
Biochemical Oxygen Demand (BOD)
The amount of oxygen required to decompose the organic matter present in sewage is called BOD.
The BOD of water during 5 days at 20º C is generally taken as the standard demand and is about 68% of the total demand.
BOD5: BOD during 5 days at 20º C
BOD5 = D.O consumed by diluted sample ×
=D.O Consumed × dilution factor
Lt = L at t = 0
Lt = Amount of organic matter Present at time t
L = Ultimate BOD(BODu)
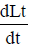 ∝Lt
∝Lt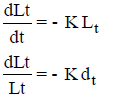
Integrating both sides, we get
Loge Lt = – Kt + C
At t = 0, Lt = L C = log L
log Lt = log L – Kt
Loge = - Kt
where Kd = K/2.3
BOD at time t = L - Lt = Yt = L – L* 10– Kdt
Yt = L [1 – 10- Kd t ] KD = De–oxygenation constant
The value of Kd changes with temperature and this relationship is approximately given by the equation
Population equivalent :
Industrial wastewaters are generally compared with per capita normal domestic wastewaters,so as to rationally charge the industries for the pollution caused by them. Thus,it indicates the strength of the industrial wastewater for estimating the treatment required at the municipal sewage treatment plant.
Relative stability :
It is the ratio of of oxygen available in effluent to the total oxygen required to satisfy its first stage BOD demand.
S = 100[1-(0.794)t20]
Or S=100[1-(0.630)t37]
where S= The relative stability,t20 and t37 represent the time in days for a sewage sample to decolourise a standard volume of methylene blue solution, when incubated at 20-degree celsius or 37 degrees celsius respectively.
|
2 videos|133 docs|55 tests
|
FAQs on Sewage & Sullage Treatment - Civil Engineering SSC JE (Technical) - Civil Engineering (CE)
| 1. What is the difference between sewage and sullage? |  |
| 2. What is aerobic decomposition in sewage and sullage treatment? |  |
| 3. What is anaerobic decomposition in sewage and sullage treatment? |  |
| 4. What does Biochemical Oxygen Demand (BOD) measure in wastewater? |  |
| 5. What are the quantity and characteristics of wastewater generated from households? |  |





















