Arboviruses, Picornaviruses and Rabies Virus Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
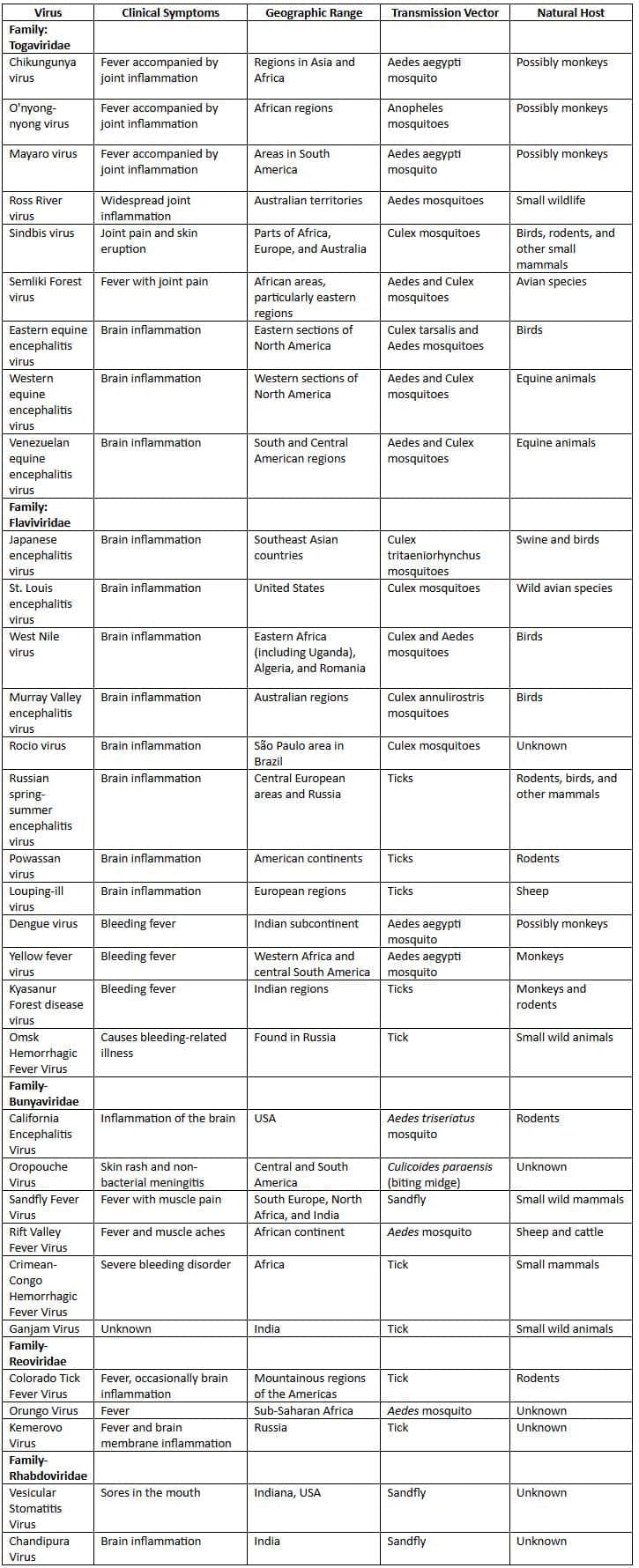
| Arboviruses |

|
| Chikungunya |

|
| Zika Virus Outbreak |

|
| Picornaviruses |

|
| Rabies Virus |

|
Arboviruses
Arboviruses are a varied group of RNA viruses that are spread by bloodsucking arthropods (insect vectors) from one animal host to another.
- Transmission cycle: Arboviruses are zoonotic, meaning they are naturally present among animals and their insect carriers.
- Humans are considered accidental hosts and do not contribute to the virus's natural cycle or spread, except in cases of urban yellow fever and dengue.
- Arboviruses found in India: More than 40 arboviruseshave been identified in India, including:
- Common: Dengue, chikungunya, and Japanese B encephalitis viruses.
- Rare: Kyasanur forest disease, West Nile, Sindbis, Crimean Congo hemorrhagic fever, Ganjam, Vellore, Chandipura, Bhanja, Umbre, Sathuperi, Chittoor, Minnal, Venkatapuram, Dhori, Kaisodi, and sandfly fever viruses.
Chikungunya
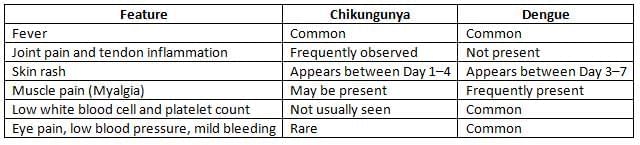
Chikungunya fever is a re-emerging disease characterized by fever with arthralgia.
- Transmission: The Aedes mosquito, mainly the Aedes aegypti, typically bites during the day. It can also be passed from mother to unborn child, but this happens very rarely. Additionally, there is a risk of transmission through blood transfusions.
- Clinical Manifestations:
- The incubation period lasts about 5 days, generally ranging from 3 to 7 days.
- The most common symptoms include fever and intense joint pain, which is caused by arthritis.
- The type of arthritis experienced is polyarticular and migratory, primarily affecting the small joints.
- Epidemiology:
- India: Chikungunya was first reported between 1963 and 1973, with instances noted in Kolkata in 1963 and in South India in 1964.
- Since then, the disease remained inactive globally from 1973 to 2005.
- Re-emergence: In 2005, Chikungunya reappeared on the Reunion Island in the Indian Ocean, and subsequently spread to India and other nations.
- India (Current Status):Chikungunya is now regularly seen in several states.
- States Affected: The states of Karnataka, Tamil Nadu, Andhra Pradesh, and West Bengal have reported a significant number of cases.
- In the years 2013 and 2014, Karnataka had the highest number of cases.
- Genotypes: There are three genotypes of Chikungunya: West African, East African, and Asian.
- Most cases in India before 1973 were linked to the Asian genotype.
- Reunion outbreak was triggered by a mutated strain, which is responsible for many recent outbreaks in India and other regions globally.
- Reasons for Re-emergence:
- New Mutation (E1-A226V): The virus has undergone a crucial mutation where the amino acid alanine at position 226 of the E1 glycoprotein gene is substituted by valine.
- New Vector (Aedes albopictus): The mutated virus exhibits a 100-fold increased infectivity to Aedes albopictus compared to Aedes aegypti.
- Laboratory Diagnosis:
- For early detection, methods such as viral isolation in mosquito cell lines and real-time RT-PCR are the most effective.
- Serum Antibody Detection: The MAC ELISA test is considered the best for serological diagnosis.
- Biological markers such as IL-1β and IL-6 are found to be elevated, while RANTES levels are decreased in cases of chikungunya infections.
Japanese B Encephalitis (JE)
Japanese Encephalitis ( JE ) is a viral infection that primarily affects the brain and is caused by the Japanese Encephalitis virus. This virus is mainly transmitted by Culex mosquitoes.
- Symptoms: The symptoms of JE can range from mild to severe and may include fever, headache, vomiting, confusion, and seizures. In severe cases, it can lead to coma and death.
- Geographic Distribution: JE is endemic in many parts of Asia, including India, Nepal, Pakistan, Thailand, Vietnam, and Malaysia. It was once common in Japan and Korea, but vaccination efforts have significantly reduced its prevalence in these countries.
- History: The disease was first identified in Japan in 1871, but it is now rare in Japan due to effective public health measures and vaccination programs.
- Transmission Cycle:The JE virus has two main transmission cycles involving animals and birds:
- Pigs → Culex mosquitoes → Pigs
- Ardeid birds (such as herons and egrets) → Culex mosquitoes → Ardeid birds
- Animal Hosts:
- Pigs are the primary amplifier host for the JE virus, where it multiplies significantly without causing illness.
- Cattle and buffaloes may attract mosquitoes, but they are not primary hosts.
- Horses are the only animals that show symptoms of the disease when infected.
- Humans are considered dead-end hosts for the virus, meaning there is no human-to-mosquito-to-human transmission cycle as seen in diseases like dengue.
- Bird Hosts: Ardeid birds, including herons, cattle egrets, and ducks, are important reservoirs for the JE virus.
Epidemiology:
- Geographical distribution: Currently, JE is mainly found in the Southeast Asian region.
- Common Countries: It is frequently seen in India, Nepal, Pakistan, Thailand, Vietnam, and Malaysia.
- Declining Incidence: Due to vaccination, the number of cases has been decreasing in Japan and Korea.
- In India: JE has been reported since 1955. It is widespread in 15 states, with Uttar Pradesh (specifically the Gorakhpur district) having the highest number of cases, followed by Assam, West Bengal, Bihar, Tamil Nadu, and Karnataka.
- Age Group: About 85% of the cases occur in children under 15 years old, although infants are not usually affected.
- Seasonal Variation: The disease is more common during the rainy season when mosquito activity is at its peak.
Clinical Manifestations:
- JE is the leading reason for outbreaks of epidemic encephalitis.
- Incubation Period: This period can last between 5 to 15 days.
- Subclinical Infection is common: JE often shows an iceberg phenomenon, where the number of actual cases is much lower compared to those with subclinical or inapparent infections, with a ratio of about 1:300 to 1:1000.
- Even during epidemics, the number of reported cases is usually just 1 to 2 in each village.
- The clinical courseof the disease can be divided into three stages:
- Prodromal Stage
- Acute Encephalitis Stage
- Late Stage with lasting neurological deficits.
Vaccine Prophylaxis:
- Live attenuated SA 14-14-2 vaccine:
- Made from the SA 14-14-2 strain.
- Derived from cell lines, typically using primary hamster kidney cells.
- Administered as a single dose under the skin, with a booster dose given after one year.
- Manufactured in China and now licensed in India.
- Part of the Universal Immunization Programme, provided to children aged 1–15 years in 83 endemic districts across four states: Uttar Pradesh, Karnataka, West Bengal, and Assam.
- Inactivated vaccine (Nakayama strain and Beijing strain):
- This vaccine is made from mouse brains and is formalin inactivated.
- It is produced at the Central Research Institute in Kasauli, India.
- Inactivated vaccine (Beijing P3 strain):
- This vaccine is also made from cell lines.
Dengue Virus
The dengue virus is the most prevalent arbovirus in India and comprises four serotypes (DEN-1 to DEN-4), with ongoing research into a potential fifth serotype (DEN-5).
Vector:
- The primary vectors responsible for its transmission are Aedes aegypti and Aedes albopictus, which are active during the daytime.
- Aedes mosquitoes become infected by feeding on individuals who are viremic, meaning they are carrying the virus, from one day before until five days after the onset of fever.
- There is an extrinsic incubation period of 8 to 10 days before Aedes mosquitoes can transmit the virus.
- Aedes mosquitoes can also pass the dengue virus to their offspring through a process called transovarial transmission.
- The transmission cycle primarily involves humans and Aedes mosquitoes, with no other animals involved.
Pathogenesis:
- A primary dengue infection occurs when a person is infected for the first time with any one serotype.
- Months or years later, a more severe form of dengue may develop, known as secondary dengue infection, caused by a different serotype.
- The severity of secondary dengue infections is due to a phenomenon called antibody-dependent enhancement (ADE), where antibodies from the first serotype help the second serotype evade the immune response.
- ADE is particularly evident when a serotype 1 infection is followed by serotype 2, which is the most severe and can lead to dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS).
- Serotype 2 is considered more dangerous than the others.
Clinical Classifications:
Traditional WHO Classification (1997) divides dengue into three clinical stages:
- Dengue Fever (DF)is marked by:
- High fever (also known as biphasic fever, break bone fever, or saddleback fever)
- Maculopapular rashes on the chest and upper limbs
- Other symptoms:
- Frontal headache
- Muscle and joint pain
- Lymph node swelling
- Loss of appetite
- Nausea and vomiting
- Dengue Hemorrhagic Fever (DHF)is characterized by:
- High continuous fever
- Hepatomegaly (enlarged liver)
- Thrombocytopenia (platelet count < 100,000/mm³)="">
- Increased hematocrit (packed cell volume) by 20%
- Signs of bleedingcan be detected by:
- Positive tourniquet test (more than 20 petechial spots per square inch in the cubital fossa)
- Spontaneous bleeding from skin, nose, mouth, and gums
- Dengue Shock Syndrome (DSS): Includes all the symptoms of DHF along with signs of shock.
WHO Classification (2009) categorizes dengue into two levels of severity:
- Dengue with or without warning signs
- Severe dengue
Geographical Distribution:
- Global Scenario: Tropical nations in Southeast Asia and the Western Pacific face the greatest risk.
- Situation in India:
- The disease is widespread in urban areas, affecting nearly 31 states and Union territories.
- The highest number of cases have been reported in Kerala, Tamil Nadu, Karnataka, Orissa, Delhi, Maharashtra, and Gujarat.
- In 2014, Maharashtra and Orissa reported the most cases.
- All four types of dengue viruses have been found in India, with DEN-1 and DEN-2 being the most common.
Laboratory Diagnosis:
- NS1 antigen detection: Tests like ELISA and ICT can identify the NS1 antigen in blood.
- Early detection: The NS1 antigen can be found from the first day of fever and stays positive for up to 18 days.
- Highly specific: This test can distinguish between different flaviviruses and dengue serotypes.
- Antibody detection:
- In a primary infection, IgM antibodies appear around 5 days after fever starts and vanish within 90 days, followed by IgG antibodies appearing after 14-21 days of illness.
- In a secondary infection, IgG antibody levels increase significantly.
- MAC: The ELISA test is the preferred method, known for its excellent sensitivity and specificity, as it can detect both IgM and IgG separately.
- Other antibody tests used before include:
- HAI: Hemagglutination inhibition test
- CFT: Complement fixation test
- Neutralization tests: Such as plaque reduction, neutralization, and microneutralization tests
- Virus detection:The dengue virus can be identified in blood from one day before symptoms start and up to five days after. This can be done by:
- Virus isolation: This can be achieved by inoculating into a mosquito cell line or a mouse.
- Detection of viral RNA: This is done using real-time RT-PCR.
Vaccine:
A live-attenuated tetravalent vaccine using a chimeric yellow fever-dengue virus (CYD-TDV) has been developed by Sanofi Pasteur. It was shown to be safe and effective in Phase III clinical trials in Latin America and has recently been approved for use in Mexico.
Yellow Fever Virus
Yellow fever is primarily found in West Africa and Central South America. It is not present in other regions of the world, including India.
- Typing: There are at least seven genotypes of the yellow fever virus identified based on genomic sequences: five in Africa and two in South America. However, there is only one serotype.
- Vector: Humans contract yellow fever through the bite of the Aedes aegypti mosquito or the tiger mosquito.
- Transmission cycle: There are two main cycles of transmission identified:
- Jungle cycle: This involves interactions between monkeys and forest mosquitoes.
- Urban cycle: This occurs between humans and city mosquitoes, specifically Aedes aegypti.
- India: Yellow fever has not yet reached India. Several reasons have been suggested to explain why yellow fever is absent in the country:
- Traveler precautions: At Indian international airports, measures are in place for travelers:
- Travelers who are unvaccinated and coming from areas where yellow fever is common will be quarantined for 6 days.
- The Breteau index, which measures the presence of Aedes aegypti, should be below one within a 400 meter radius of the airport.
- Dengue antibodies: Antibodies from dengue can offer some protection against yellow fever. However, being vaccinated against yellow fever does not provide protection against dengue.
- Traveler precautions: At Indian international airports, measures are in place for travelers:
- Clinical signs: The incubation period for yellow fever is about 3 to 6 days. Common symptoms include:
- Jaundice: This is why the disease is called yellow fever.
- Mid-zonal necrosis: This includes the presence of Councilman bodies.
- Intranuclear inclusions: These may be visible inside liver cells and are referred to as Torres bodies.
Yellow Fever 17D Vaccine
- Type: Live attenuated vaccine
- Source: Derived from the allantoic cavity of a chick embryo
- Safety: No risk of encephalitis, unlike the earlier used Dakar vaccine
- Production in India: Manufactured at the Central Research Institute (CRI), Kasauli
- Cold Chain: Must be maintained strictly between -30°C and +5°C
- Form: Available in lyophilised form and requires reconstitution with diluents like physiological saline before use
- Reconstitution: Once reconstituted, the vaccine should be used within ½ hour
- Dosage:. single dose is administered subcutaneously
- Onset of Immunity: The vaccine becomes effective within 7 days of administration and generally provides long-lasting immunity
- Validity of Certificate: certificate is issued after 10 days of vaccination and requires reimmunisation every 10 years
- Interaction with Cholera Vaccine: These vaccines should not be given together; a gap of 3 weeks is required
- Contraindications:
- Children under 6 months and those under 9 months during an epidemic
- Pregnancy (except during an outbreak)
- HIV infection
- Allergy to egg
Kyasanur Forest Disease Virus (KFD)
- The KFD virus was first identified in 1957 in monkeys from the Kyasanur Forest located in the Shimoga district of Karnataka, India.
- Vector: Hard ticks, specifically Haemaphysalis spinigera.
- Hosts:
- Reservoirs: Rats and squirrels.
- Amplifier hosts: Primarily monkeys, although KFD can also affect humans.
- Humans: Considered an incidental host and a dead-end for the virus.
- Clinical Manifestation in Humans: The incubation period for KFD in humans ranges from 3 to 8 days. The disease initially presents as hemorrhagic fever, which is followed by a second phase of meningoencephalitis.
- Seasonality: KFD cases are more commonly reported during the dry months, specifically from January to June. This increase coincides with heightened human activity in forested areas.
- Endemic Regions: KFD is endemic to five districts in Karnataka: Shimoga, North Canara, South Kannada, Chikkamagaluru, and Udupi.
- Largest Outbreak: The most significant outbreak of KFD occurred in 1983–84. Since the introduction of the KFD vaccine in 1999, there has been a decline in cases, with only focal cases being reported currently.
- The KFD vaccine is highly recommended for use in endemic areas of Karnataka, particularly in villages located within 5 kilometers of known endemic foci.
Zika Virus Outbreak
Microbiology
- The Zika virus is a member of the Flaviviridae family and belongs to the Flavivirus genus.
- It is a single-stranded RNA virus, related to other viruses such as dengue, yellow fever, Japanese encephalitis, and West Nile viruses.
History
- The Zika virus was first identified in 1947 in the Zika Forest of Uganda, which is how it got its name.
- Initially, the virus circulated among humans without causing significant harm.
- From its discovery until 2007, only 14 confirmed cases were reported worldwide.
- The first outbreak of Zika virus occurred in 2007 in the Yap Islands, primarily involving the Aedes hensilli mosquito species.
- During this outbreak, there were 49 confirmed and 59 probable cases of Zika virus infection.
- Monkeys are considered reservoirs for the Zika virus, harboring the virus in their bodies.
Epidemiology
Transmission:
- Mosquito Transmission: The Zika virus is primarily spread by the Aedes aegypti mosquito, but it can also be transmitted by other types of Aedes mosquitoes, including Aedes albopictus.
- Mother-to-Child Transmission: The virus can be passed from a mother to her baby through the placenta, which is common during the first trimester. While it is rare, transmission can also occur during delivery.
- Sexual Transmission: There are cases of the Zika virus being transmitted through sexual contact. As of August 26, 2016, there have been 17 recorded cases. Transmission can happen from:
- Asymptomatic males to their female partners.
- Symptomatic females to their male partners.
- The virus can stay longer in the semen of infected individuals.
Current Outbreak (2015-2016):
- The Zika virus outbreak started in April 2015 in Brazil.
- It quickly spread to various countries in South America, Central America, and the Caribbean.
- There have also been reported cases in Europe, the United States, and Australia.
- As of January 30, 2017:
- There were 174,665 suspected cases and 528,157 confirmed cases, with 18 reported deaths.
- In Brazil alone, there were nearly 109,596 suspected cases and 200,465 confirmed cases.
- The countries with the next highest number of cases after Brazil include Puerto Rico, Colombia, and Mexico.
- In February 2016, the WHO declared the Zika virus outbreak a public health emergency of international concern.
Situation in India:
No cases of the Zika virus have been reported in India so far. However, there is some evidence that people in India have previously tested positive for Zika virus antibodies. Since the mosquito that carries the virus is present in India, there is a possibility that the country may face an outbreak in the future.
Clinical Manifestations
- Incubation Period: The exact incubation period is unknown but is typically a few days to 1 week.
- Symptomatic vs. Asymptomatic Cases: The majority of cases are asymptomatic, with a ratio of approximately 5:1 asymptomatic to symptomatic cases.
- Symptoms of Zika Fever: When symptoms do occur, Zika fever usually causes mild symptoms such as fever, rash, and conjunctivitis.
- Congenital Transmission: Congenital transmission of the Zika virus can result in newborns being born with microcephaly, a condition where the baby's head is significantly smaller than expected, potentially leading to developmental issues.
- Guillain-Barré Syndrome: There have been very few reported cases of Guillain-Barré syndrome associated with Zika virus infection, with some cases documented in French Polynesia.
Lab Diagnosis
- IgM ELISA: This test is available for diagnosing Zika virus but may cross-react with antibodies from dengue virus, leading to false-positive results.
- Plaque-Reduction Neutralization Test: This test is considered more specific for diagnosing Zika virus infections compared to IgM ELISA.
- RT-PCR: Reverse transcription polymerase chain reaction (RT-PCR) is performed on patients who are acutely ill and suspected of having a Zika virus infection. This test helps detect the viral RNA in the patient's samples.
Treatment and Vaccine
- No effective treatment or vaccine is currently available.
- Intensive research is being conducted for vaccine development by various companies, including Bharat Biotech from India.
- A new investigational Zika vaccine, created by NIAID and NIH, has started phase 1 clinical trials. This vaccine includes a genetically engineered plasmid, which is a small, circular piece of DNA that carries the Zika virus protein.
- The only available treatments are symptomatic treatments, such as replacing lost fluids and taking pain relievers like acetaminophen.
Prevention Measures for Zika Virus
- Pregnancy Advisory: Countries like Brazil, Colombia, Ecuador, El Salvador, and Jamaica are advising women to delay pregnancy until more is known about the risks associated with Zika virus infection.
- Travel Restrictions: Pregnant women from countries, including India, are facing travel restrictions to Zika-affected areas to prevent potential infection.
- Mosquito Control: Mosquito control measures in Zika-affected areas are similar to those used for preventing dengue, aiming to reduce the population of mosquitoes that can transmit the virus.
- Post-Infection Precautions: Individuals infected with Zika virus should avoid mosquito bites for the first week of their illness to prevent further transmission.
- CDC Recommendations: The Centers for Disease Control and Prevention (CDC) provide guidelines on sexual activity and pregnancy restrictions for individuals who have traveled to Zika-affected areas. These include:
- Men: Consider using condoms or abstaining from sex for at least 6 months after travel (if asymptomatic) or from the onset of symptoms (or Zika diagnosis).
- Women: Consider using condoms or abstaining from sex for at least 8 weeks after travel (if asymptomatic) or from the onset of symptoms (or Zika diagnosis).
- Throughout Pregnancy: Use condoms consistently for all types of sex or avoid sexual activity throughout the pregnancy.
WHO Guideline: Algorithm for Diagnosis of Zika Virus Infection
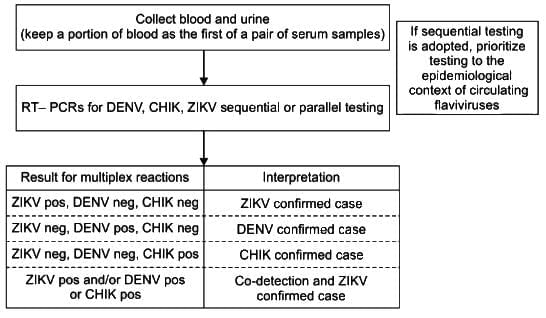 A negative result for any PCR test does not conclusively rule out the infection
A negative result for any PCR test does not conclusively rule out the infection
Proposed testing algorithm for suspected cases of arbovirus infection identified within seven days of onset of symptoms
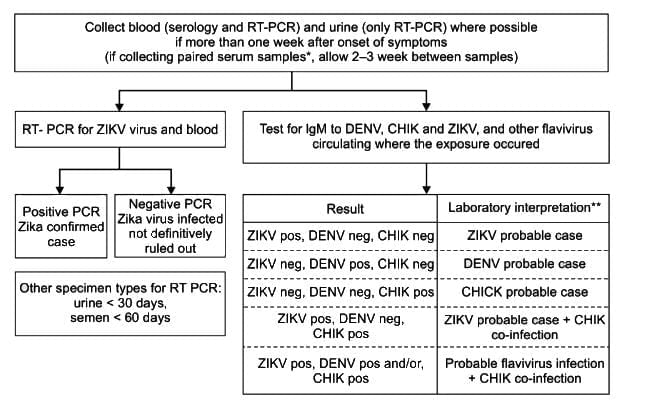
- For period serum sample, a four-fold rise in IgM in the absence of a rise in antibody titre to other flavivirus is further evidence of recent Zika virus infection
- Final interpretation of result should be done in conjunction with clinical presentation
Proposed testing algorithm for suspected cases of arbovirus infection more than one week after onset of symptoms
Picornaviruses
Picornaviruses are small viruses, measuring about 28–30 nanometers in size, and they do not have a protective outer layer. These viruses are primarily divided into two main groups:
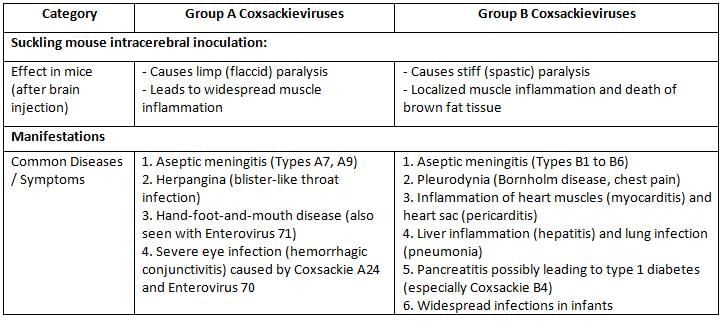
- Enteroviruses: Enteroviruses are transmitted through the fecal-oral route, but surprisingly, they do not usually cause problems in the intestines. Instead, they have systemic effects on the body. Some examples of enteroviruses include:
- Polio: There are three different types (serotypes) of poliovirus.
- Coxsackie A: There are 24 different types, numbered from 1 to 24.
- Coxsackie B: There are six different types, numbered from 1 to 6.
- Echovirus: There are 33 different types, numbered from 1 to 33.
- Parechovirus: There are three different types, numbered from 1 to 3.
- Enteroviruses: Types 68 to 71.
- Enterovirus 72 has been studied in relation to Hepatitis A, but it is important not to classify it as such without proper context.
- Rhinoviruses: Rhinoviruses are spread through respiratory droplets and are the primary cause of the common cold.
Polioviruses
Serotypes of Poliovirus
- Type 1: Caused by the Brunhilde and Mahoney strains. This is the most common type responsible for epidemics and is currently the only type found worldwide.
- Type 2: Caused by the Lansing and MEF-1 strains. This type is the most antigenic, making it easier to eliminate. There have been no natural cases since 1999, and it is the most common type found in vaccine-derived poliovirus (VDPV) strains.
- Type 3: Caused by the Leon and Saukett strains. There have been no natural cases since 2013, but this type is the most common cause of vaccine-associated paralytic poliomyelitis (VAPP).
Pathogenesis
- Transmission: The main way the virus spreads is through the fecal-oral route, but it can also spread through inhalation or contact with the eyes.
- Receptor: The virus enters host cells by attaching to the CD155 receptors found on the surface of these cells.
- Spread to CNS/spinal cord: The virus spreads to the central nervous system (CNS) and spinal cord mainly through the bloodstream (hematogenous spread). It can also spread directly through nerves, especially after a tonsillectomy, where it travels via the glossopharyngeal nerve in the tonsillar area.
- Site of action: The virus primarily targets motor nerve endings, particularly the anterior horn cells in the spinal cord, which can lead to flaccid paralysis.
- Neuron degeneration: The first change in neurons is the degeneration of Nissl bodies, which are clusters of ribosomes usually found in the cytoplasm of neurons.
- Pathological changes: The extent of pathological changes is often greater than the areas affected by paralysis.
Clinical Manifestations
The incubation period typically lasts between 7 to 14 days. The disease can show up in four different forms:
- Inapparent infection: Most cases (91-96%) do not show any symptoms after infection.
- Abortive infection: About 5% of patients experience mild symptoms like fever and general discomfort.
- Nonparalytic Poliomyelitis: This occurs in 1% of patients and is presented as aseptic meningitis.
- Paralytic Poliomyelitis:This is the least common form, affecting less than 1% of those infected. It is characterized by:
- Asymmetric acute flaccid paralysis (AFP) that descends in nature.
- Proximal (closer to the center of the body) muscles are affected before distal (further from the center) muscles, causing paralysis to start at the hip and move towards the limbs. This results in the characteristic tripod position, where a child sits with flexed hips and both arms extended backward.
- Involvement can occur in spinal, bulbospinal, and bulbar areas.
- Cranial nerves may be affected, but there is typically no loss of sensation.
Risk factors: Paralytic disease is more likely to occur in:
- Older children and adults, pregnant women, and those who engage in heavy muscular activities.
- Tonsillectomy can increase the risk of bulbar poliomyelitis.
- Intramuscular (IM) injections may raise the risk of paralysis in the affected limb.
Laboratory Diagnosis
- Specimen collection: Samples can include blood (within 3-5 days), throat swabs (up to 1 week), cerebrospinal fluid (CSF), and feces (up to 6-8 weeks).
- Virus isolation:Primary monkey kidney cell lines are used for this purpose. The growth of the virus can be seen through various methods, including:
- Cytopathic effect (CPE): This is described as the shrinkage and degeneration of the entire cell layer.
- Antigen detection: The virus can be identified and typed through neutralization with specific antisera.
- Gene detection: Specific genes can be identified using PCR (Polymerase Chain Reaction).
- Stool cultures are recommended for monitoring acute flaccid paralysis (AFP) and for laboratory confirmation of the virus.
- Culture results from CSF, serum, or throat swabs are less common but can indicate the presence of the disease.
- Antibody detection: Testing for neutralizing antibodies and complement fixation test (CFT) antibodies is performed.
Types of Polio Vaccines
There are two types of polio vaccines: Oral Polio Vaccine (OPV) and Inactivated Polio Vaccine (IPV).
Vaccine Vial Monitor (VVM):
- It is a tool to check the stability and potency of Oral Polio Vaccine (OPV) and to ensure the cold chain is working efficiently.
- The tool is a heat-sensitive label that is placed on the OPV vial. It features a ring with two circles:
- The outer circle is blue.
- The inner square is white.
- According to WHO grading:
- The outer circle will always remain blue.
- The inner square can be:
- White (grade I)
- Light blue (grade II)
- Blue (grade III)
- Black/Purple (grade IV)
- The OPV can be used if it is at grade I or II, but it should be thrown away if it reaches grade III or IV.
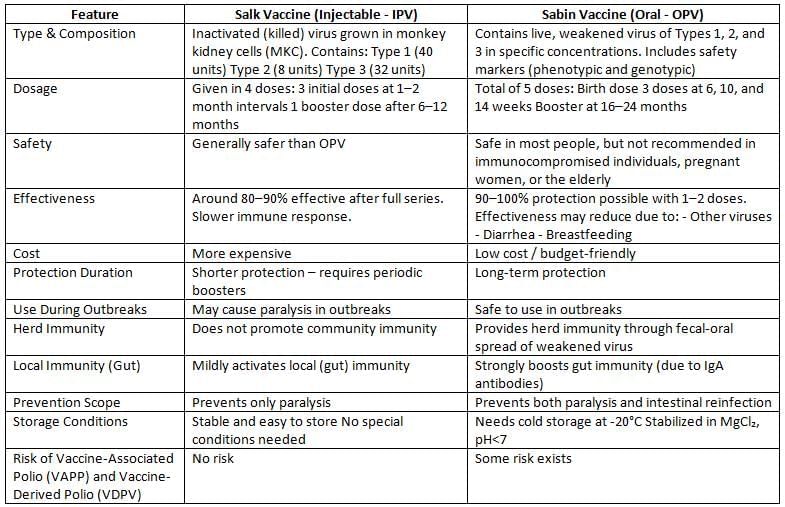
Vaccine-Induced Cases (VAPP and VDPV)
Vaccine-Associated Paralytic Poliomyelitis:
VAPP cases occur following the administration of Oral Polio Vaccine (OPV) and are caused by specific OPV strains that undergo mutation.
- These VAPP strains are closely related to the OPV strains, differing by less than 1%.
- VAPP cases are prevalent in regions where OPV is widely used.
- VAPP can affect both OPV recipients and their close contacts.
- However, VAPP strains do not have the ability to circulate within the community and do not trigger outbreaks due to high levels of population immunity.
- The VAPP rate is approximately one case per 2.5 million doses of OPV.
- VAPPhappens more often:
- After the first dose of OPV compared to later doses.
- In individuals with primary immunodeficiency disorder (risk increases by 3000 times).
- The main serotype linked to VAPP is Sabin type 3 (60%), followed by type 2.
Vaccine-Derived Polioviruses (VDPVs):
VDPV isolates exhibit a greater genetic divergence from their parent OPV strains at the VP1 sequence, which facilitates their prolonged replication and dissemination.
- The genetic divergence of VDPVs from parent OPV strains is approximately:
- More than 1% for Sabin types 1 and 3
- More than 0.6% for Sabin type 2
- Isolates with genetic differences below these thresholds are categorized as OPV-like isolates.
- VDPV isolates are clinically and phenotypically indistinguishable from wild polioviruses due to the restoration of their neurovirulence and the reversal of their attenuation markers.
- The majority of VDPV isolates are of Sabin type 2 (90%), followed by type 1, as wild type 2 strains have not circulated since 1999.
VAPP and VDPV
- VAPP strains are OPV-like isolates differing from OPV by less than 1%.
- VDPVs differ from OPV by more than 1% for serotypes 1 and 3 and more than 0.6% for Sabin type 2.
- VDPVs can be categorized as:
- Circulating VDPVs (cVDPVs)
- Immunodeficiency-associated VDPVs (iVDPVs)
- Ambiguous VDPVs (aVDPVs)
Types of VDPVs
- Circulating VDPVs (cVDPVs):
- These strains can spread from person to person within the community.
- They can cause outbreaks in areas where the oral polio vaccine (OPV) coverage is low.
- They pose a similar risk to the community as wild polioviruses.
- Since the year 2000, cVDPV outbreaks have been reported in 18 countries, including India, with most cases (87.1%) linked to type 2.
- In 2015, over 27 cases of VDPVs were found around the world, with Madagascar having the highest number of cases.
- Immunodeficiency-associated VDPVs (iVDPVs):
- These are found in individuals with primary immunodeficiency disorders.
- They do not cause disease but can shed iVDPVs for many years.
- iVDPVs show more genetic variation than cVDPVs, with some strains differing by more than 10%.
- The level of genetic difference increases with the length of the infection.
- Unlike cVDPVs, high OPV coverage does not prevent infections caused by iVDPVs.
- Ambiguous VDPVs (aVDPVs):
- These are diverse strains; they may be cVDPVs for which only one case has been identified or sewage samples from developed countries with an unknown source, likely iVDPV.
Epidemiology:
- Reservoir: Humans are the only known reservoir, and most cases are asymptomatic.
- Clinical Cases: For every clinical case, there may be about 1,000 children and 75 adults with subclinical cases.
- No Chronic Carriers: There are no long-term carriers, but immunodeficient individuals can shed the virus for extended periods.
- Source of Infection: Infections come from infectious materials like stool and oropharyngeal secretions.
- Age Group: Younger children and infants are more at risk than adults, although in developed nations, older children are increasingly affected.
- Infectious Period: Patients can spread the virus in their feces from 7 to 10 days before symptoms appear and continue for 2 to 3 weeks after, sometimes up to 3 to 4 months.
Polio Eradication:
Poliomyelitis is close to being eradicated due to extensive global immunization efforts.
Pulse Polio Immunization (PPI):
- Two rounds of PPI are carried out every year during winter, spaced 6 weeks apart, where all children under five are vaccinated with OPV, regardless of their previous vaccination status.
- PPI doses of OPV are considered additional doses and do not replace those given as part of the regular national immunization schedule.
Polio Situation in the World:
- Endemic Countries: Currently, polio is endemic in only three countries: Pakistan, Afghanistan, and Nigeria (which was declared polio-free in 2015 but became endemic again in 2016).
- Vulnerable Countries: Countries that are no longer infected with wild poliovirus but are still at risk of international spread include Cameroon, Equatorial Guinea, Ethiopia, Iraq, Israel, Nigeria, Somalia, and the Syrian Arab Republic.
- Reported Cases (as of January 2017):
- In 2016, there were only 35 cases of wild poliovirus (wPV) reported, down from 71 cases in 2015, and 4 cases of cVDPV (27 cases in 2015).
- Pakistan had the highest number of natural cases, while Lao People's Democratic Republic was the only non-endemic country to report 3 cases of cVDPV.
- Currently, all natural cases of polio are linked to type-1.
- Type-2 and type-3 polio cases have not been reported since 1999 and 2013, respectively.
- India has been declared polio-free since 2014.
- The last natural case of polio in India was found three years ago, in January 2011.
- Global Polio Eradication Initiative (GPEI): The GPEI launched the ‘Eradication and Endgame Strategic Plan’ (2013–2018) aiming for global polio eradication by 2018.
Endgame Strategic Plan (2013–2018)
- Objectives: Interrupt poliovirus transmission, strengthen immunization systems, and withdraw OPV gradually while switching to IPV.
- IPV Introduction: One dose of IPV was to be introduced by the end of 2015, replacing the third dose of OPV.
- Serotype-2 Withdrawal: Trivalent OPV will be replaced by Bivalent OPV (serotypes 1 and 3) six months after starting IPV (mid-2016).
- IPV-Only Schedule: An IPV-only schedule is planned by 2019, replacing OPV completely.
- Poliovirus Containment: Ensuring poliovirus containment and certifying the world as polio-free by the end of 2018.
- Legacy Planning: Utilizing the infrastructure, funding, manpower, and expertise from the global polio programme to support other health initiatives post-eradication.
Rabies Virus
Morphology
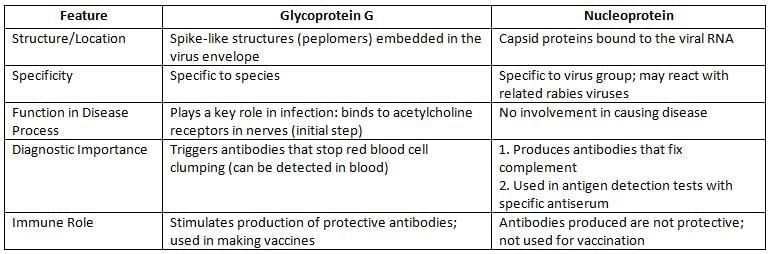
- Shape: The virus has a bullet-like shape and is covered by an envelope.
- Envelope Features: The envelope contains spikes made of glycoprotein, which act as antigens.
- Nucleocapsid: This part of the virus is made of nucleoprotein and has a helical structure.
- Genetic Material: The virus carries negative sense single-stranded RNA (ssRNA).
- Antigens: Rabies virus has two main antigens:
- Glycoprotein G
- Nucleoprotein
Pathogenesis
- Transmission:
- Rabies virus primarily spreads to humans through bites from infected animals, with dog bites being the most common.
- Other animal bites that can transmit the virus include those from monkeys, sheep, goats, etc. (bites from rats and humans are excluded).
- Human-to-human transmission is theoretically possible but extremely rare.
- Non-bite exposures, although rare, may occur through:
- Licking an abrasion or mucosal surface,
- Inhaling aerosols from infected bats,
- Corneal transplantation.
- Route of Spread:
- The rabies virus replicates in muscle tissue.
- It binds to nicotinic acetylcholine (ACh) receptors at the neuromuscular junctions.
- The virus then spreads centripetally along peripheral motor nerves to the dorsal root ganglia of the spinal cord.
- From there, it infects neurons in the brain, particularly in the brainstem and limbic system.
- The virus also spreads centrifugally via sensory and autonomic nerves to various organs, including the cornea, salivary glands, skin, and other tissues.
Clinical Manifestations
- Incubation period: 1 to 3 months (20 to 90 days)
- Shorter incubation: This period is usually shorter in children and for bites on the upper limbs, as well as in shorter individuals compared to those with leg bites or taller people.
- First symptom: The initial sign is nerve pain at the site of the bite.
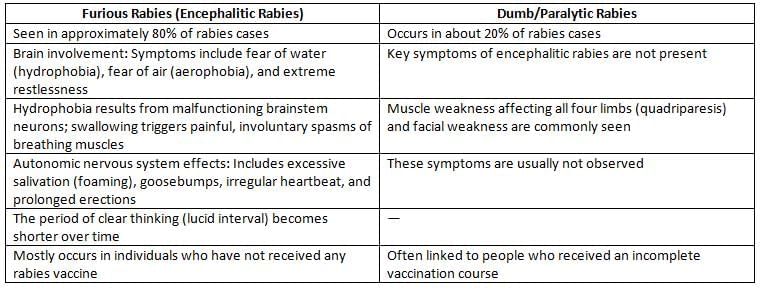
- Phases of the illness:
- Short prodromal phase: A brief early stage of the disease.
- Acute neurologic phase:This stage can appear in two types:
- Encephalitic rabies: A serious form affecting the brain.
- Dumb rabies: A less aggressive form with different symptoms.
- Coma and death: This can happen within 14 days for encephalitic rabies and within 30 days for dumb rabies.
Laboratory Diagnosis
- Rabies antigen detection
Laboratory diagnosis of rabies typically involves the following methods:- Direct IF test identifies rabies nucleoprotein antigens in samples.
- The best sample to use is the hair follicle from the nape of the neck, as it is the most sensitive.
- A corneal impression smear can test positive in the later stages of the disease, but it has a sensitivity of only 30%.
- Viral Isolation
- Mouse inoculation: This method involves injecting directly into the brains of baby mice.
- Cell lines: The best options for this type of research are mouse neuroblastoma cell lines and baby hamster kidney (BHK) cell lines.
- Antibody detection: Finding antibodies in the cerebrospinal fluid (CSF) is more important than in the blood serum. This is because serum antibodies show up later and could also be present due to vaccination.
- Mouse neutralization test (MNT)
- Rapid fluorescent focus inhibition test (RFFIT)
- Fluorescent antibody virus neutralization (FAVN)
- Indirect fluorescence assay (IFA)
- Hemagglutination inhibition test (HAI)
- Complement fixation test (CFT)
- Viral RNA detection:
- Reverse transcription-polymerase chain reaction (RT-PCR) is currently the most sensitive and specific test for diagnosing rabies.
- Negri body detection:
- Finding Negri bodies is a key sign for diagnosing rabies after death. However, they may not be found in 20% of cases, so their absence does not rule out rabies.
- Negri bodies are eosinophilic inclusions found inside the cell, with distinctive basophilic inner granules.
- They are well-defined, round to oval shapes, measuring about 2 to 10 micrometers.
- The main locations for these bodies are the neurons in the cerebellum and hippocampus, but they can also be found in cortical and brainstem neurons.
- Common stains used: Histological stains like H and E, and Sellers stains (which include basic fuchsin and methylene blue).
Prevention of Human Rabies
Prevention of human rabies involves post-exposure prophylaxis (PEP), which includes local wound care and both active and passive immunization.
Local Treatment:
Local treatment for potential rabies exposure includes:
- Promptly cleaning the wound by scrubbing it with soap and water, followed by the application of antiseptics.
- Bite wounds should not be sutured immediately.
- For category II and III bites, confirmation of whether the animal is rabid is needed for 10 days.
Passive Immunisation:
- Human rabies immune globulin (HRIG): Administered at a dose of 20 IU/kg, with the maximum injected locally; the remainder is given intramuscularly in the gluteal region. HRIG is indicated only for category III bites.
- Equine rabies immunoglobulin (ERIG): Administered at a dose of 40 IU/kg. ERIG is less commonly used due to the risk of serum sickness since it is derived from horses.
Active Immunisation (Rabies Vaccine):
- Neural Vaccines: These vaccines have poor immunogenicity and a risk of causing encephalitis, so they are no longer used. Examples include the Semple vaccine (derived from infected sheep brain), BPL vaccine (prepared in Coonoor), and infant mouse brain-derived vaccine.
- Non-neural Vaccines:These cell line-derived vaccines are currently recommended and include:
- Purified chick embryo cell (PCEC) vaccine.
- Purified Vero cell (PVC) vaccine.
- Human diploid cell (HDC) vaccine.
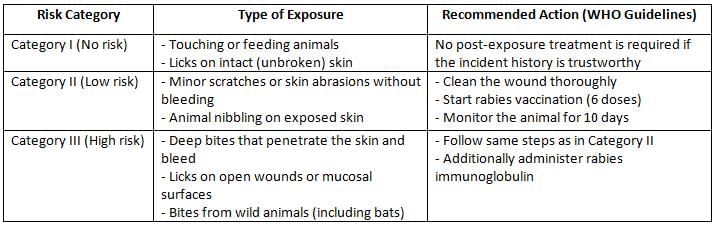
National Guideline on Rabies Prophylaxis
(Adapted from National Center for Disease Control, India)
Regimen for Post-Exposure Prophylaxis:
- IM regimen or Essen regimen (1-1-1-1-1): Five doses (0.5 or 1 ml each) are given on days 0, 3, 7, 14, and 28, with a booster after 90 days. Day 0 is the date of the first vaccine dose, not the exposure date.
- ID Regimen (or Thai Red Cross Schedule) (2-2-2-0-2): This involves injecting 0.1 ml of the reconstituted vaccine at two sites per visit on days 0, 3, 7, and 28.
- Potency: A single intramuscular dose should have a minimum potency of 2.5 IU.
- Site of injection:
- The deltoid region is the best site. The gluteal region is not recommended because fat slows down the absorption of the antigen.
- For infants and young children: The anterolateral part of the thigh is the preferred site.
Regimen for Pre-Exposure Prophylaxis:
- Recommended for high-risk groups such as veterinarians, animal handlers, and travelers to rabies-free areas.
- Three doses of vaccine are given on days 0, 7, and 28, with a booster every 2 years.
- Antibody levels should be checked every 6 months for the first 2 years, and then every 2 years after that.
- A booster dose is given if the antibody level is less than 0.5 IU/ml.
Regimen for Post-Exposure Prophylaxis for Previously Vaccinated People:
- For a severe bite or if the titer is unknown: 3 doses of vaccine on days 0, 3, and 7.
- For a less severe bite or if the titer is greater than 0.5 IU/ml: 2 doses of vaccine on days 0 and 3.
Epidemiology
- Rabies is a disease that affects both wild and domestic animals all over the world. It can occur in two forms: enzootic (regularly found) and epizootic (outbreaks).
- Globally, rabies is found in more than 150 countries. Each year, around 55,000 people die from rabies. India has the highest number of cases, with about 20,000 deaths annually.
- The virus that causes rabies is present in the saliva of an infected dog. This virus can be detected 3-4 days before symptoms appear and continues until the dog dies.
- A rabies-free area is defined as a country or region where no rabies cases have been reported in the last two years. Some examples include:
- Worldwide:
- Australia
- Antarctica
- Britain
- Iceland
- Ireland
- China (Taiwan)
- Cyprus
- Japan
- Malta
- New Zealand
- India:
- Andaman and Nicobar Islands
- Lakshadweep
- Worldwide:
- To control urban rabies, it is important to remove stray dogs and vaccinate at least 80% of the dogs in a given area.
|
75 docs|5 tests
|
FAQs on Arboviruses, Picornaviruses and Rabies Virus Chapter Notes - Microbiology - NEET PG
| 1. What is Chikungunya and how is it transmitted? |  |
| 2. What are the historical milestones in the identification of Chikungunya? |  |
| 3. What are the clinical manifestations of Chikungunya? |  |
| 4. How is Chikungunya diagnosed in the laboratory? |  |
| 5. What are the current treatment options and preventive measures for Chikungunya? |  |














