Atmospheric Pollution Chapter Notes | Environmental Management for Grade 11 PDF Download
| Table of contents |

|
| Introduction |

|
| Air Pollution |

|
| Acid Rain |

|
| Global Warming |

|
| Ozone Layer |

|

Introduction
The environment includes the air, water, and land around us. It changes from place to place due to various factors. Human activities like industries and transport release harmful substances called pollutants, which cause pollution—unwanted changes that harm humans, animals, and plants. While nature can also create pollutants, human-made ones damage the environment more quickly. The word "pollution" comes from the Latin word pollutes, meaning "made dirty."
On the Basis of Their Origin (Sources), Pollutants Are of Two Types: Natural and Man-Made
- Natural pollutants: These pollutants occur naturally in the environment, such as volcanic ash, pollen, or dust storms.
- Man-made pollutants: These pollutants are a result of human activities, such as industrial waste, emissions from vehicles, and chemicals used in agriculture.
Natural Sources of Air Pollutants
- Volcanoes: When volcanoes erupt, they release large quantities of air pollutants, including sulphur dioxide and fine particulate matter, into the atmosphere.
- Decaying Vegetation: The decomposition of plants and organic materials by tiny microbes leads to the release of pollutants like nitrous oxide into the air.
- Forest Fires: Forest fires are a significant source of carbon monoxide, a harmful gas that contributes to air pollution.
- Winds and Dust Storms: Winds and dust storms can transport small particles such as sand and dust into the atmosphere, contributing to air pollution.
- Ozone: Ozone itself is a major air pollutant that can adversely affect air quality.
Man-Made Sources of Air Pollutants
- Automobiles: Vehicles powered by diesel or petrol emit harmful substances when these fuels are not burned completely. The pollutants released include carbon monoxide, sulfur dioxide, hydrocarbons, nitrogen oxides, and small particles that may contain heavy metals like lead.
- Factories: Industrial facilities contribute to air pollution by releasing significant amounts of carbon dioxide, sulfur dioxide, nitrogen dioxide, and particulate matter into the atmosphere.
- Industrial Processes: Different operations within industries release various air pollutants specific to the processes involved.
- Examples:
- Power plants are known to emit carbon monoxide, sulfur dioxide, ash, and smoke as byproducts of electricity generation.
- Fertiliser industries contribute to air pollution by releasing nitrogen oxides and ammonia into the atmosphere during their production processes.
Air Pollution
- Air pollution occurs when harmful substances are present in the air, making it unsafe for humans, plants, and animals.
- For air to be healthy, it needs to contain essential gases like oxygen and nitrogen. Pollution disrupts this balance and harms the atmosphere.
- Air pollution is caused by harmful gases such as sulfur oxides, nitrogen oxides, carbon oxides, and hydrocarbons. It also involves tiny particles like dust, smoke, mist, spray, fumes, aerosols, and particulate matter.
Gaseous Components of 'Ordinary' Dry Air (Non-Polluted)
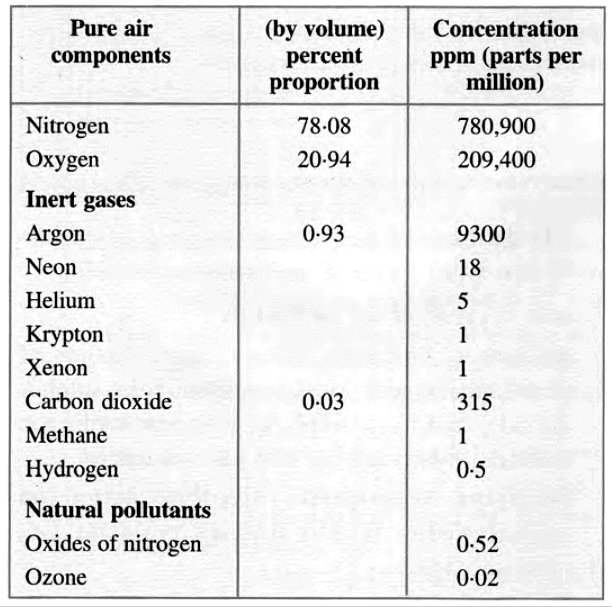
Gaseous Pollutants and Their Effects
- Sulfur Dioxide: This gas is harmful to both crop yields and human lungs.
- Hydrogen Sulfide: It slows down crop growth and irritates human eyes.
- Fluorides: These can damage plants and crops and may also affect human teeth and bones.
- Nitrogen Oxides: These gases primarily cause breathing problems and do not have a direct link to cancer.
- Carbon Monoxide: This gas interferes with haemoglobin's ability to transport oxygen in the body.
- Environmental Tobacco Smoke: This is a known cause of lung cancer.
- Lead: Lead from vehicles using tetraethyl lead disrupts the body's metabolism.
- Cotton Dust: This leads to lung fibrosis, while smoke particles can trigger asthma and other lung diseases.
- Smog: Smog is harmful and irritating; it reduces visibility, causes breathing difficulties, and can lead to suffocation.
The other main pollutants together contribute to more than 90% of global air pollution, and they are as follows:
- Nitrogen oxides (N2O, NO, NO2)
- Hydrocarbons (primarily methane, CH4)
- Sulphur oxides (SO2, SO3)
- Hydrogen sulphide (H2S)
- Carbon monoxide (CO)
- Particulates (fine solid particles and liquid droplets)
Oxides of Nitrogen as Air Pollutants
- Nitric oxide (NO) and nitrogen dioxide (NO2) enter the atmosphere in the following ways:
- During the burning of fuels in furnaces, temperature increases, and nitrogen and oxygen in the air combine to form oxides of nitrogen.
- When fuel burns in an internal combustion engine, oxides of nitrogen are produced, and they enter the atmosphere as exhaust gases from automobiles.
- Nitric acid is formed by the reaction between atmospheric nitrogen and oxygen during electric discharge, which happens during thunderstorms when there is lightning.
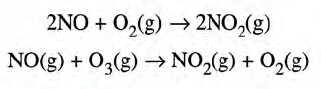
Harmful Effects of the Oxide of Nitrogen
- It causes irritation in mucous membranes like in the nose, throat, and eyes.
- Large amounts of NO2 can cause serious lung diseases.
- Nitrogen dioxide causes serious injury to plants; it damages plant leaves.
- In sunlight, nitrogen dioxide forms photochemical smog.
- Photochemical smog causes eye irritation, asthma attacks, and throat infections.
Compounds of Sulphur as Pollutants
- Compounds of sulphur like sulphur dioxide (SO2) and hydrogen sulphide (H2S) are pollutants.
- Hydrogen sulphide (H2S) is produced by decaying organic matter, such as rotten vegetables, and by sewage and certain industrial operations.
- H₂S is also produced by industrial processes like coal and oil.
Harmful Effects of Hydrogen Sulphide
- It causes nausea and irritates the eye and throat.
- Hydrogen sulphide destroys the vegetative matter.
- Sulphur dioxide (SO2) and sulphur trioxide (SO3) are produced by processes like burning fuels containing coal and oil.
- They are also produced by metallurgical processes involving sulphide ores.
Harmful Effects of Oxides of Sulphur
- It causes headaches, vomiting, and even death due to breathing problems.
- It destroys vegetation and weakens building materials/constructions.
- It mixes with smoke and fog to form smog, which is very harmful.
Carbon Monoxide (CO) as an Air Pollutant
- Carbon monoxide (CO) is released when fuels are incompletely burned in various settings such as homes, factories, and vehicles.
- CO is an extremely toxic gas.
- When inhaled, carbon monoxide passes through the lungs and enters the bloodstream.
- Once in the bloodstream, it prevents haemoglobin (a protein in red blood cells) from transporting oxygen to different parts of the body.
- Haemoglobin has a stronger attraction to carbon monoxide than to oxygen, which means that even small quantities of CO can be dangerous.
- CO reduces the blood's capacity to carry oxygen by forming a compound called carboxyhemoglobin with haemoglobin.
- The chemical reaction can be summarised as: Haemoglobin + CO → Carboxyhemoglobin.
- The heart and brain are the most vulnerable to oxygen deprivation, and they suffer severe consequences from exposure to carbon monoxide.
- In high concentrations, carbon monoxide can be fatal by disrupting normal brain function.
Monitoring carbon monoxide levels and taking measures to reduce its emissions are crucial for protecting human health and the environment.
Control of Carbon Monoxide Pollution
It can be controlled in the following ways:
- By switching over from internal combustion engines to electrically powered cars.
- Although the latter would transfer the source of pollution to power companies, it is easy to control the pollution created by power companies.
- Many pollution control devices are now installed in cars to help reduce pollution by burning gasoline completely.
- Complete combustion of gasoline produces only carbon dioxide and water vapor:
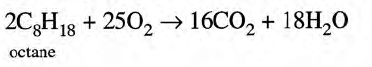
- By using substitute fuels for gasoline: Natural gas in both compressed (CNG) and liquefied forms (LNG) is now increasingly being used as fuel. Alcohols are other feasible substitutes.
Using Substitute Fuels for Gasoline
- Natural gas in both compressed (CNG) and liquefied forms (LNG) is now increasingly being used as fuel.
- Alcohols are other feasible substitutes.
Using Catalytic Converters
- Nitrogen oxide is reduced to nitrogen and oxygen in the presence of a finely divided platinum or palladium catalyst.
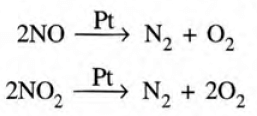
- Carbon monoxide changes into carbon dioxide in the presence of finely divided platinum as catalyst.
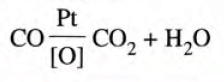
Acid Rain
- The term "acid rain" is used to describe all types of rain, snow, fog, or dew that are more acidic than normal water.
- Normal rain is only slightly acidic, having a pH of about 5.6, because carbon dioxide reacts with it to form weak carbonic acid.
Equation: CO2 + H2O → H2CO3.
Composition of Acid Rain
- Acid rain is primarily caused by the presence of strong acids like sulfuric acid and nitric acid in polluted air.
- These acids form when nitrogen oxides and sulfur oxides react with moisture in the atmosphere.
- The pH levels of acid rain typically range from 4.0 to 5.0, indicating a higher level of acidity compared to normal rainwater.
Causes of Acid Rain
- Nitric Acid Formation: Nitric acid forms when nitrogen and oxygen in the atmosphere react, particularly during thunderstorms and lightning.
- Oxides of Nitrogen from Engines: Internal combustion engines produce oxides of nitrogen. At high temperatures, nitrogen monoxide (NO) forms from the reaction N₂ + O₂ → 2NO. Then, nitrogen monoxide is converted to nitrogen dioxide (NO₂) through the reaction 2NO + O₂ → 2NO₂.
- Formation of Nitric Acid: Nitrogen dioxide reacts with water to produce nitric acid and nitrous oxide, as shown in the equation 2NO₂ + H₂O → HNO₃ + NO.
- Sulfuric Acid from Coal: Sulfuric acid originates from coal containing 2 to 4% sulfur, commonly used in power plants.
- Production of Sulfur Dioxide: Burning coal generates sulfur dioxide (SO₂). Similarly, burning petroleum products releases sulfur dioxide. Other sources of sulfur dioxide include chemical plants and metal-processing industries, such as those for copper, lead, and zinc.
- Formation of Sulfur Dioxide and Trioxide: Sulfur, a non-metal found in fossil fuels like coal and fuel oil, combines with oxygen when these fuels are burned, forming sulfur dioxide (SO₂) and sulfur trioxide (SO₃). The reaction S + O₂ → SO₂ illustrates the formation of sulfur dioxide, and 2SO₂ + O₂ → 2SO₃ shows the conversion to sulfur trioxide.
- Reaction with Water: Sulfur dioxide and sulfur trioxide react with water to produce sulfuric acid, a major contributor to acid rain, as shown in the equation SO₂ + O₂ + H₂O → H₂SO₄.
Impact of Acid Rain
- Soil Chemistry: Acid rain negatively impacts soil chemistry by leaching essential nutrients such as calcium and magnesium from the soil. This process reduces the fertility of the soil, making it less suitable for plant growth.
- Plant Health: Acid rain causes plants to lose vital nutrients, leading to damage in their leaves. This nutrient loss can impair the overall health and growth of plants.
- Ecosystem Impact: Acid rain has a severe impact on ecosystems by increasing the acidity of water bodies like lakes and rivers. This change in water chemistry can harm aquatic life and disrupt the balance of these ecosystems.
- Damage to Buildings: Acid rain can cause significant damage to buildings and structures because it contains acids that dissolve various materials. This can lead to structural weakening and deterioration over time.
- Taj Mahal: The Taj Mahal is experiencing serious problems due to the corrosion of metals caused by acid rain. For example, acid rain accelerates the corrosion of iron in structures such as gates and iron frames, leading to further damage.
- Human Health: Acid rain also affects human health. In high concentrations, it can impact a person’s breathing. Sulphur dioxide, a component of acid rain, irritates the upper respiratory tract and the respiratory system. Even in low concentrations, it can affect small particles and dust inhaled from the air.
Reducing the Impact of Acid Rain
- To mitigate the effects of acid rain, it is essential to tackle its primary causes, which involve reducing the emissions of sulfur oxides and nitrogen oxides released during the combustion of fossil fuels.
- One effective approach is to use low-sulfur coal or oil, which can significantly decrease the amount of sulfur dioxide produced during burning.
- Additionally, the installation of scrubbers can help reduce emissions by capturing harmful gases such as sulfur dioxide and nitrogen oxides before they are released into the atmosphere.
- A typical scrubber for removing sulfur dioxide operates by spraying a fine mist of water that interacts with the gas, facilitating its absorption and thus reducing its contribution to acid rain formation.
- It is important to recognise that acid rain poses serious threats to the environment and human health, damaging forests, harming aquatic ecosystems, and potentially affecting human well-being.
Global Warming
- Global warming refers to the rise in the Earth's average temperature due to an increase in greenhouse gases. These gases come from both natural sources and human activities.
- The greenhouse effect happens when certain gases in the atmosphere trap heat from the sun, making the Earth warmer.
- The main gases responsible for this effect are carbon dioxide (CO2 ), methane (CH4 ), nitrous oxide (N2O), and water vapour.
- These gases are released into the atmosphere when fossil fuels like coal, oil, and natural gas are burned, along with various other human activities.
Greenhouse Effect
- The Earth gets its heat from the sun, and a portion of this heat is trapped by gases in the atmosphere. This phenomenon is known as the greenhouse effect.
- The term "greenhouse effect" comes from glass structures called greenhouses, which are used to grow plants in cooler climates.
- In a greenhouse, sunlight passes through the glass, warming the interior, and the heat is kept inside.
- Similarly, gases like carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and water vapour trap the sun's heat, helping to maintain the Earth's warmth.
- If these gases were not present, the Earth's heat would escape into space, causing the planet to become very cold.
This process is crucial for sustaining life on Earth by regulating temperature.
Greenhouse Gases
Greenhouse gases are responsible for trapping heat in the Earth's atmosphere, leading to the greenhouse effect. The main gases involved are carbon dioxide (CO₂), water vapour, nitrogen oxides, methane, ozone, and chlorofluorocarbons. Here’s how each gas contributes to the greenhouse effect:
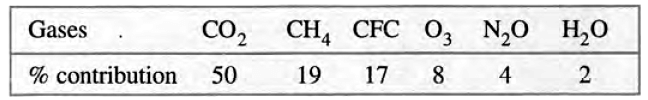
Sources of greenhouse gases: The major sources of greenhouse gases are:
Carbon Dioxide (CO₂)
Carbon dioxide is one of the most significant greenhouse gases. It is released into the atmosphere through a variety of natural and human-made processes.
- Burning of Fossil Fuels: When fossil fuels such as coal, petroleum (oil), and natural gas are burned for energy in power plants, vehicles, and industries, carbon dioxide is produced as a by-product.
Example: Running a diesel generator or driving a petrol car emits CO2. - Industrial Processes: Certain manufacturing activities release CO2 into the atmosphere.
Example: Production of cement and lime involves heating limestone (calcium carbonate), which releases carbon dioxide. - Fermentation Processes: In industries like alcohol production, yeast ferments sugars to produce ethanol and carbon dioxide.
- Biological Decay: When plants and organic matter decompose naturally, they release carbon dioxide as a part of the decay process.
- Respiration: All living organisms – including humans, animals, and even plants – exhale carbon dioxide during the process of respiration.
Water Vapour
Water vapour is the most abundant greenhouse gas, though its concentration varies greatly depending on temperature and location.
- Burning of Hydrocarbons: When substances that contain hydrogen and carbon (like fossil fuels) burn, they produce water vapour and carbon dioxide.
- Example: Combustion of natural gas (methane) in stoves or vehicles releases water vapour.
Hydrocarbon + Oxygen → Carbon Dioxide + Water Vapour
Oxides of Nitrogen
- Oxides of nitrogen, like nitrous oxide (N2O), are greenhouse gases that trap heat.
- These gases are made during the burning of fuels like coal, oil, and natural gas in vehicles and power plants.
- Plants also release small amounts of nitrous oxide into the air.
- Oxides of nitrogen are harmful because they help cause global warming and also form acid rain.
Methane
- Methane (CH4) is a powerful greenhouse gas that plays a significant role in trapping heat within the Earth's atmosphere.
- This gas is produced when organic materials, such as plants and animals, decompose in the absence of oxygen. This process commonly occurs in environments like swamps and rice fields.
- Additionally, methane is released during the burning of fossil fuels, including coal, oil, and natural gas.
- Ruminant animals, such as cows, also contribute to methane emissions as the gas is produced in their stomachs during the digestion process.
- Methane is significantly more effective than carbon dioxide at trapping heat, which makes it exceptionally harmful to the environment.
- Moreover, methane contributes to air pollution, degrading air quality and exacerbating climate change.
Mechanism of Green House Effect
The greenhouse effect is a natural process that warms the Earth's surface. This happens when sunlight reaches the Earth and is reflected back into space, but some of it is trapped by greenhouse gases in the atmosphere.
- The greenhouse effect happens in three main steps: radiation, trapping, and heating.
- The sun sends visible and ultraviolet (UV) radiation to the Earth.
- Most UV radiation is absorbed by the ozone layer, while the rest reaches the Earth's surface.
- The Earth absorbs some of this radiation and emits infrared (IR) radiation back into the atmosphere.
- Greenhouse gases like carbon dioxide (CO2), methane (CH4), water vapor, and nitrous oxide (N2O) trap this IR radiation, preventing it from escaping into space.
- This trapped heat causes the Earth's temperature to rise, a process known as global warming.
- Over time, the increasing amount of greenhouse gases leads to higher temperatures, resulting in issues like melting ice and rising sea levels.
Effects of Global Warming
- Climate change leads to a rise in the Earth's temperature, causing a range of issues.
- It results in the melting of ice in regions such as the Arctic and Antarctic, contributing to rising sea levels.
- Increased sea levels pose a threat to low-lying areas, including islands and coastal cities, which may face flooding.
- Climate change also disrupts weather patterns, leading to more frequent and intense storms, droughts, and floods.
- This phenomenon can have detrimental effects on wildlife and plants, as their natural habitats are altered, and some species may struggle to survive.
- In countries like India, Bangladesh, and the Maldives, rising sea levels endanger homes and agricultural land.
Ways of Reducing Global Warming
- Use bicycles or public transport instead of cars to reduce fuel use.
- Plant more trees because they take in CO₂ and give out oxygen.
- Stop burning dry leaves, wood, or garbage because it releases harmful gases.
- Use clean fuels like CNG instead of coal or diesel to reduce pollution.
- Try to use energy wisely, like turning off lights and fans when not needed.
- Help others understand the importance of keeping the air clean.
Ozone Layer
Ozone is a light, bluish gas found in the upper layer of the atmosphere (stratosphere). Ozone is formed by the action of ultraviolet rays of the sun on oxygen.
Function of Ozone in the Atmosphere
- Ozone in the stratosphere is about 16 km above the Earth's surface.
- It absorbs most of the harmful UV rays coming from the sun.
- UV rays can harm living things by causing skin cancer and damaging plants.
- The ozone layer breaks down UV rays into oxygen: O3 + → O2 + O.
- Ozone helps keep the Earth's temperature balanced by controlling how much UV radiation reaches the surface.
Destruction of the Ozone Layer
- The ozone layer is getting destroyed because of harmful chemicals called chlorofluorocarbons (CFCs).
- CFCs are used in refrigerators, air conditioners, and spray cans.
- When CFCs are released into the air, they break down ozone molecules into oxygen, which cannot protect us from UV rays.
- The reaction is: CF2Cl2(g) → CF2Cl(g) + Cl(g), then Cl(g) + O3(g) → ClO(g) + O2(g).
- Another reaction is: ClO(g) + O(g) → Cl(g) + O2(g).
- These reactions lead to a reduction in ozone levels, thinning the ozone layer and allowing more UV rays to reach the Earth's surface.
- The depletion of the ozone layer can have severe consequences for ecosystems and human health, including an increase in skin cancer rates and damage to marine life.
Chemicals Responsible for the Destruction of the Ozone Layer
- Chlorofluorocarbons (CFCs) are the primary substances responsible for damaging the ozone layer.
- These chemicals are often found in everyday items such as refrigerators, air conditioners, spray cans, and fire extinguishers.
- When CFCs are released into the atmosphere, they rise to the stratosphere, where they break down and release chlorine atoms.
- The chlorine atoms react with ozone (O 3 ), converting it into oxygen (O 2 ).
- The chemical reaction involved is as follows:
CF2 Cl2 (g) → CF2 Cl(g) + Cl(g)
Cl(g) + O3 (g) → ClO(g) + O2 (g) - Another reaction that occurs is:
ClO(g) + O(g) → Cl(g) + O2 (g) - This process continues, leading to the ongoing destruction of ozone as chlorine (Cl) interacts with more ozone molecules.
Understanding the impact of CFCs on the ozone layer is essential for protecting our environment and ensuring the well-being of future generations.
Fuel of Planes
- Planes burn fuels that release harmful gases like nitric oxide (NO) and nitrogen dioxide (NO2) into the air.
- These gases also destroy ozone in the stratosphere.
- The reaction is: NO(g) + O3(g) → NO2(g) + O2(g).
- This reduces the amount of ozone in the air, making the ozone layer thinner.
Harmful Effects of Ozone
- Ground-level ozone is harmful and has a chlorine-like smell.
- It leads to respiratory issues in both humans and animals.
- It damages plants and trees, hindering their growth and development.
- Ground-level ozone weakens the rubber in car tyres, increasing the risk of tyre damage.
- Ground-level ozone is formed when pollutants from vehicles and industries react with sunlight.
Moreover, the depletion of the ozone layer can result in increased UV radiation, which adversely affects human health and disrupts the environmental balance.
Depletion of Ozone
- Depletion of ozone means the ozone layer in the stratosphere is getting thinner.
- This happens because of chemicals like CFCs and nitrogen oxides that break down ozone into oxygen.
- The reaction is: Cl(g) + O3(g) → ClO(g) + O2(g).
- A thinner ozone layer allows more harmful ultraviolet (UV) rays to reach the Earth.
- UV rays can lead to serious health issues such as skin cancer, eye problems, and can also harm plants and animals.
List of Major Air Pollutants: Their Origin and Health Impact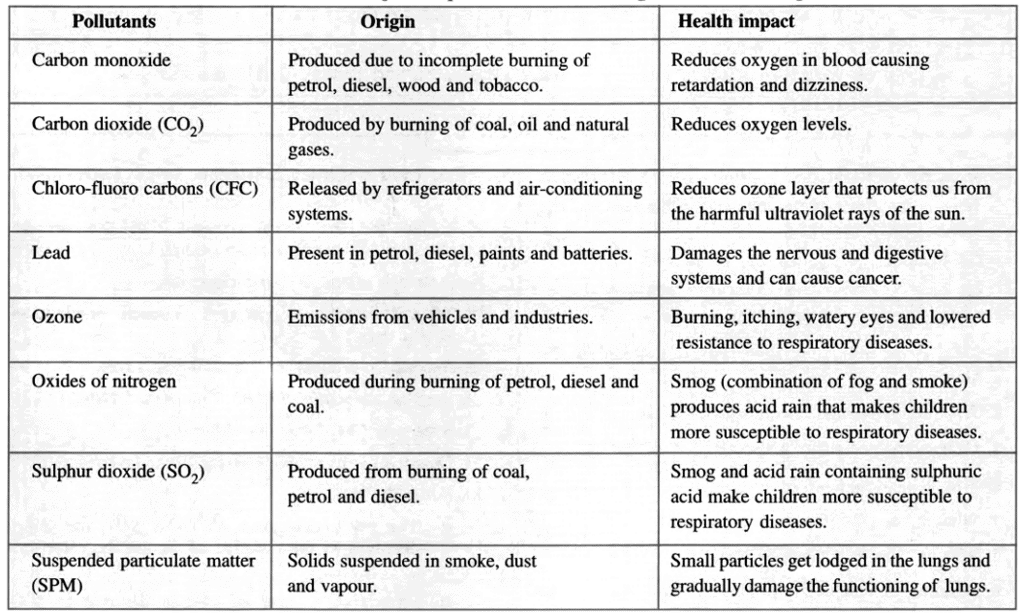
|
39 docs|2 tests
|
FAQs on Atmospheric Pollution Chapter Notes - Environmental Management for Grade 11
| 1. What are the main causes of air pollution? |  |
| 2. How does acid rain form and what are its effects? |  |
| 3. What is global warming and what are its main contributors? |  |
| 4. What is the role of water vapor in the atmosphere? |  |
| 5. Why is ozone depletion a concern and what causes it? |  |




















