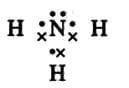Atomic Structure and Chemical Bonding Chapter Notes | Chemistry for SSS 1 PDF Download
Introduction
The chapter "Atomic Structure and Chemical Bonding" introduces the fundamental concepts of atoms, their structure, and how they combine to form compounds. It explores the historical development of atomic theories, the discovery of subatomic particles like electrons, protons, and neutrons, and the models proposed by scientists like Dalton, Thomson, Rutherford, and Bohr. The chapter also explains how atoms achieve stability through chemical bonding, either by transferring electrons (electrovalent bonding) or sharing electrons (covalent bonding). Additionally, it covers concepts like isotopes, atomic number, mass number, and the octet rule, which govern the chemical behavior of elements.- Maharshi Kanada (6th Century B.C.) proposed that matter consists of tiny, indestructible particles called "paramanus" (atoms) that combine to form "anu" (molecules).
- Each paramanu has specific properties and cannot exist independently.
- Greek philosopher Democritus (460–370 B.C.) named these particles "atoms," meaning indivisible.
- John Dalton (1808) developed the first scientific atomic theory, suggesting atoms are the smallest units of matter that participate in chemical reactions.
Dalton’s Atomic Theory
- Matter is made of small, indivisible particles called atoms.
- Atoms cannot be created or destroyed.
- Atoms of the same element are identical but differ from atoms of other elements.
- Atoms combine in small numbers to form molecules.
- Atoms of different elements combine in simple ratios to form compounds.
- Atoms are the smallest units that take part in chemical reactions.
- Note: Modern research shows most of Dalton’s postulates are incorrect, except that atoms participate in chemical reactions.
Definition of an Element
- An element is a pure substance made of only one type of atom.
- It cannot be broken down or formed from simpler substances by ordinary physical or chemical methods.
- Example: Carbon cannot be split into simpler substances by heating or electricity.
- Note: Radioactive decay or high-energy nuclear reactions can transform one element into another.
Definition of an Atom
- An atom is the smallest particle of an element that shows all its properties.
- It may not exist independently but participates in chemical reactions.
- Example: Dividing a piece of zinc into smaller pieces eventually results in atoms that retain zinc’s properties.
Constituents of an Atom
Discovery of Electrons- William Crookes found that gases at very low pressure (0.01 mm Hg) conduct electricity when a high voltage (10,000 volts) is applied, producing cathode rays.
- Cathode rays originate from the cathode (negative electrode) and move toward the anode (positive electrode).
- J.J. Thomson studied cathode rays using a discharge tube and concluded they consist of negatively charged particles called electrons.
Properties of Cathode Rays
- Travel in straight lines from cathode to anode.
- Produce greenish-yellow fluorescence on a soda-glass screen.
- Are deflected by electric fields toward the positive plate, indicating a negative charge.
- Produce X-rays when striking hard metallic targets like tungsten.
- Can penetrate matter.
- Cause ionization of gases they pass through.
- Have a constant charge-to-mass ratio (e/m = 1.76 × 10¹¹ coulomb/kg).
- Produce a shadow of an opaque object and can rotate a light paddle wheel.
Thomson concluded that :
- Cathode rays consist of negatively charged particles, now called electrons.
- [The name ‘electron,’ meaning ‘atom of negative electricity,' was given by Johnson Stoney].
- These negatively-charged particles are an integral part of all atoms.
- Electrons have both definite mass and definite electric charge, both of which are independent of the nature of the gas in the discharge tube.
Properties of Electrons
- Identical mass and charge regardless of the source.
- Constituent of all atoms.
- Mass is 1/1837 of a hydrogen atom (9.108 × 10⁻³¹ kg).
- Carry a unit negative charge (–1.602 × 10⁻¹⁹ coulombs).
- Extremely small, with a radius less than 1 × 10⁻¹⁵ m.
- Denoted as ₋₁e⁰ (0 for mass, –1 for charge).
Discovery of Protons
- Goldstein observed canal rays (positive rays) moving from anode to cathode in a discharge tube with a perforated cathode.
- These rays were named canal rays because they pass through holes in the cathode.
Properties of Anode Rays
- Travel in straight lines.
- Consist of minute material particles, producing mechanical effects.
- Positively charged particles called protons.
- Deflected by electric and magnetic fields in the opposite direction to cathode rays.
- Produce fluorescence on a zinc sulphide screen.
- Charge-to-mass ratio varies with the gas used, highest for hydrogen.
Properties of Protons
- A proton carries a unit positive charge of +1, equivalent to 1.602 × 10⁻¹⁹ coulombs.
- Its mass is 1 atomic mass unit (a.m.u.), which is 1837 times the mass of an electron (1.672 × 10⁻²⁴ g).
- Protons are located in the central part of an atom, known as the nucleus.
After the Discovery of Electrons and Protons
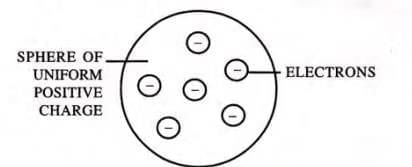
J.J. Thomson proposed the "plum pudding" model of the atom.
According to this model:
- The atom is a sphere with a uniform positive charge, and electrons are embedded within it.
- The total positive charge balances the total negative charge, making the atom electrically neutral.
- The mass of the atom is evenly distributed throughout the sphere.
Discovery of Nucleus
- Rutherford (1911) bombarded a thin gold foil with alpha particles (doubly charged helium ions, He²⁺).
- Observations:
- Most alpha particles passed straight through.
- Some were slightly deflected.
- Very few were deflected by large angles or bounced back.
- Heavy metals like platinum show similar results, but light nuclei like lithium may be pushed aside by alpha particles.
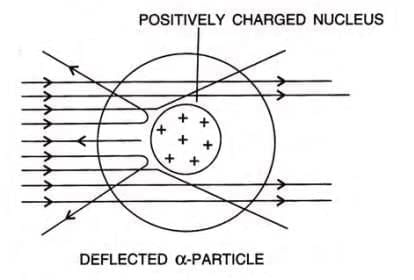
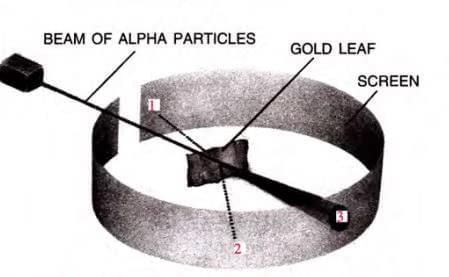
Later, Rutherford Generalized the Findings of the Alpha Particle Scattering Experiment and Proposed a Model of the Atom Known as Rutherford’s Atomic Model
Note: Heavy metals like platinum will exhibit the same behavior with alpha (α) particles as observed with gold foil. However, if lighter nuclei such as lithium are used, the fast-moving alpha particles might push the light nucleus aside and may not be reflected back.
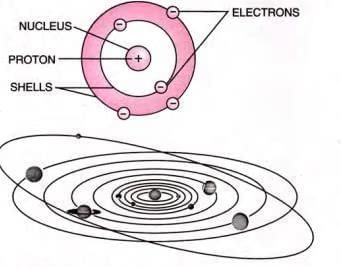
Rutherford’s Atomic Model
- Atom has a large empty space (extranuclear space).
- Contains a dense, positively charged nucleus at the center with most of the atom’s mass.
- Nucleus is very small compared to the atom’s size.
- Electrons revolve around the nucleus in circular orbits.
- Atom is electrically neutral (equal number of protons and electrons).
- Force of attraction between electrons and nucleus is balanced by centrifugal force.
- Similar to the solar system, with the nucleus as the sun and electrons as planets.
Drawback of Rutherford’s Model
According to the classical laws of mechanics and electromagnetism, an electrically charged particle in motion, like an electron orbiting the nucleus, should continuously emit radiation. This means the electron would lose energy as it moves around the nucleus. Consequently, it should gradually spiral inward and eventually collide with the nucleus, leading to the complete collapse of the atom If this were true, atoms would be highly unstable, which contradicts our understanding since matter is generally stable. Therefore, we conclude that Rutherford’s model does not adequately explain the stability of an atom.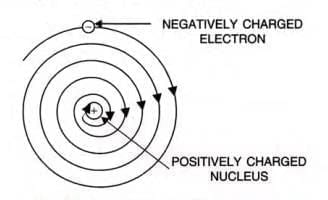
Bohr’s Atomic Model
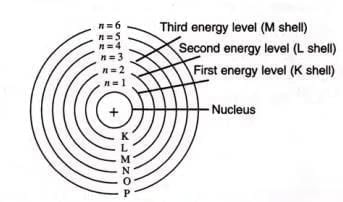
- Proposed by Niels Bohr (1913) to explain atom stability.
- Electrons revolve in fixed orbits (shells) with specific energy levels.
- Electrons neither gain nor lose energy while in an orbit.
- Electrons jump to higher orbits by gaining energy or drop to lower orbits by losing energy.
- Shells are labeled K, L, M, N (or I, II, III, IV), with K being closest to the nucleus and having the least energy.
Discovery of Neutrons
- Helium’s atomic mass (4 amu) suggested additional particles besides protons (2 amu).
- Chadwick (1932) discovered neutrons by bombarding beryllium with alpha particles:
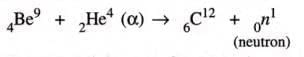
These particles are found to be neutral, so named neutrons.
A neutron is a sub-atomic particle or fundamental particle of an atom with no charge and mass almost equal to the mass of the proton i.e. hydrogen atom. Neutron is denoted by 0N1. The superscript 1 represents its mass and subscript 0 represents its electrical charge.
Properties of Neutrons
- Not deflected by magnetic or electric fields (electrically neutral).
- Neutrons are neutral, with mass equal to protons (1.676 × 10⁻²⁴ g, 1 amu).
After the discovery of electrons, protons and neutrons (subatomic particles) it was found that all atoms have a similar following basic structure.
- Electrons are negatively charged particles that are found outside the nucleus.
- Protons are positively charged particles that are found in the nucleus of an atom.
- Neutrons are electrically neutral particles that are also found in the nucleus. Neutrons are slightly heavier than protons.
Atom – Its Structure
- Consists of a nucleus (protons and neutrons) and orbits (electrons).
- Properties of subatomic particles:

There are two structural parts ot an atom, the nucleus and the empty space in which there are imaginary paths called orbits.
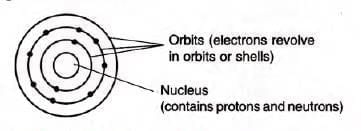
Nucleus: The protons and neutrons [collectively called nucleons] are found in the central part or nucleus of the atom.
Orbits: It is the imaginary path where electrons revolve around the nucleus of the atom.
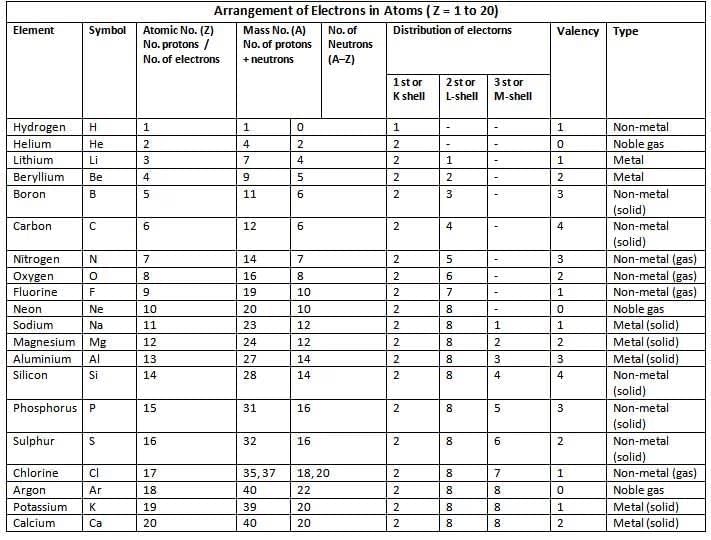
Atomic Number (Z)
- Number of protons in the nucleus of an atom.
- Equal to the number of electrons in a neutral atom.
- Represents the total positive charge in the nucleus.
- Denoted by Z.
The atomic number of an element is the number of:
- Protons present in the nucleus of its atom.
- Electrons present in its neutral atom.
- Positive charge in the nucleus of its atom.
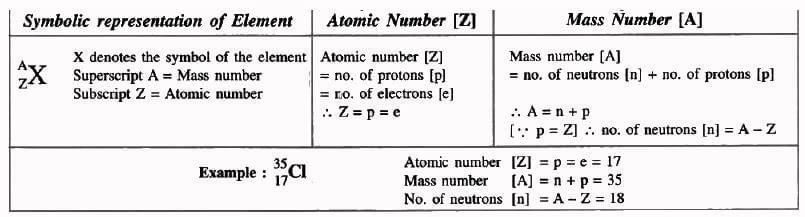
Mass Number (A)
- Total number of protons and neutrons in the nucleus.
- Denoted by A.
- Number of neutrons = A – Z.
Distribution of Electrons in Orbits – Bohr-Bury Scheme
Electronic configuration is the distribution of electrons in different shells.The following rules are followed for writing the number of electrons in different energy levels or shells.
- Maximum electrons in a shell = 2n², where n is the shell number

- Inner shells must be filled before outer shells.
- Outermost shell cannot have more than 8 electrons (except helium, with 20 electrons).
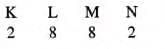
- The M shell can hold a maximum of 18 electrons, but only 8 electrons are typically filled in it. This is because elements achieve stability once they have 8 electrons in their outermost shell.
- The outermost shell of a chemically stable atom can contain a maximum of 8 electrons, with the exception of the helium atom. Helium has only one shell and can therefore hold a maximum of just 2 electrons.
Main Features of Atomic Structure
Magnesium has an atomic number of 12 and a mass number of 24. Therefore, it contains 12 protons and (24 – 12 =) 12 neutrons. It issurrounded by 12 electrons, which are distributed across different shells as follows:
- K shell (or I shell) = 2 electrons
- L shell (or II shell) = 8 electrons
- M shell (or III shell) = 2 electrons
Thus, its electronic configuration is 2, 8, 2.
In lighter elements up to argon (atomic number 18), each inner shell is completely filled before any electron occupies an outer shell. However, in
elements heavier than argon, the situation changes. Although the third shell can hold up to 18 electrons, the fourth shell begins to be filled after the third shell has only 8 electrons.
For example, potassium (atomic number 19) has the electronic configuration:

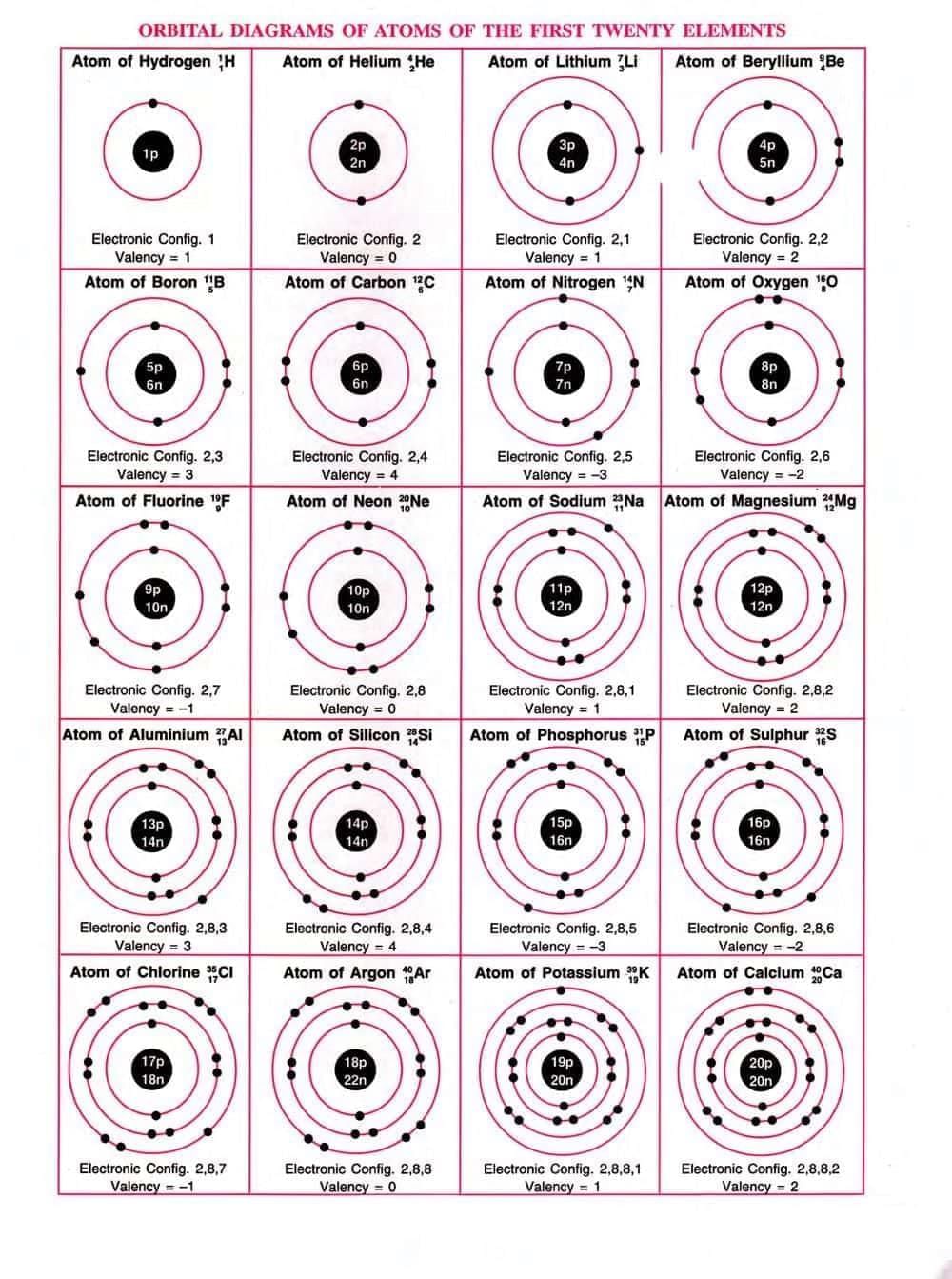
Main features of the structure ot atoms :
- Atoms of all the elements (except hydrogen) are made up of three fundamental (sub-atomi particles: electrons, protons and neutrons. (Hydrogen atom lacks neutrons).
- The nucleus is located at the centre of the atom. It contains protons and neutrons, which accou for the total mass of that atom.
- The nucleus is positively charged due to the presence of protons in it.
- The electrons are outside the nucleus and have negligible mass.
- The number of electrons in an atom is equal to the number of protons in it, hence the atom electrically neutral.
- The electrons revolve rapidly in fixed circular paths around the nucleus. These circular paths a called energy levels or shells or orbits.
- The atoms of different elements contain different numbers of electrons, protons and neutrons.
Valence Electrons
The outermost shell of an atom is referred to as its valence shell, and the electrons in this shell are known as valence electrons.or example, hydrogen has just one electron in its valence shell, making it its only valence electron. Similarly, carbon has 4 electrons in its valence shell, so it has 4 valence electrons.
The chemical properties of elements are determined by their valence electrons, as these are the electrons that participate in chemical reactions. Elements that have the same number of valence electrons exhibit similar properties, while those with different numbers of valence electrons display different chemical properties.
Elements with 1, 2, or 3 valence electrons (except hydrogen) are metals. These elements can lose electrons to form positively charged ions, known as cations.
Reason for Chemical Activity of an Atom
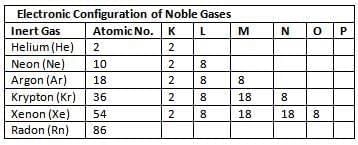
- Noble gases (except helium) have 8 electrons in their outermost shell (octet), making them stable and non-reactive.
- Helium has 2 electrons (duplet) in its K shell, also stable.
- Chemically active atoms have incomplete valence shells (less than 8 electrons, or 2 for hydrogen).
- Kossel and Lewis (1916) proposed the octet rule: atoms combine to achieve 8 electrons in their valence shell (or 2 for hydrogen, duplet rule).
- Atoms achieve stability by gaining, losing, or sharing electrons.
- Chemical bond: Force of attraction between atoms forming a molecule.
Isotopes
- Atoms of the same element with the same atomic number but different mass numbers due to varying neutrons.
- Have identical chemical properties due to the same electronic configuration.
- Differ in physical properties like density and boiling point.
- Examples:
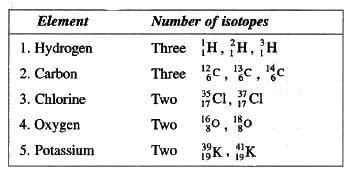
Structures of Isotopes
Hydrogen
Hydrogen has three isotopes: protium, deuterium, and tritium. They all have the same atomic number (1) but different mass numbers (1, 2, and 3, respectively). Protium has no neutrons, deuterium has 1 neutron, and tritium has 2 neutrons. These isotopes differ in their mass numbers because of the varying number of neutrons, even though the number of protons (1) and electrons (1) remains the same.
Carbon
Carbon (Atomic Number 12 and Mass Number 14)
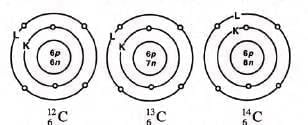
These isotopes have atomic numbers of 6 and mass numbers of 12, 13, and 14, respectively. They are represented as ¹²₆C, ¹³₆C, and ¹⁴₆C. For ¹⁴C, the isotope has 14 – 6 = 8 neutrons. Some isotopes, such as ¹⁴C, are radioactive.
Uses of Isotopes
- ⁶⁰₂₇Co: Used in radiotherapy for cancer treatment.
- ¹⁴₆C: Determines the age of historical and geological materials.
- ²³⁵₉₂U: Fuel in nuclear reactors.
- ¹³¹₅₃I: Treatment of goitre.
Fractional Atomic Masses
An atom can have a fractional atomic mass. For example, the atomic mass of chlorine is 35-5. The reason for fractional atomic mass is that the atomic mass of an element represents the weighted average of all the naturally occurring isotopes of that element. For example, any natural sample of chlorine contains 75% of
, i.e. these isotopes are in the ratio of 3 : 1 respectively. Thus, average atomic mass of chlorine is calculated as 35 x 3 + 37 x 1/4 = 35-5. Therefore atomic mass of chlorine atom is 35.5.
Isobars
- Atoms of different elements with the same mass number but different atomic numbers.
- Have different chemical and physical properties.
- Examples:

Electrovalent (or Ionic) Bond
- Atoms of metals with 1, 2, or 3 valence electrons can give electrons to non-metal atoms with 5, 6, or 7 valence electrons to form an electrovalent compound.
- After giving or taking electrons, both atoms get the same electron arrangement as the nearest noble gas, making them stable.
- A metal atom that loses electrons becomes a positively charged ion called a cation.
- A non-metal atom that gains electrons becomes a negatively charged ion called an anion.
- An ion is an atom that becomes charged by gaining or losing electrons.
- Metals that easily lose electrons to form positive ions are called electropositive elements.
- Example: Sodium loses 1 electron: Na – e⁻ → Na⁺ (cation).
- Non-metals that easily gain electrons to form negative ions are called electronegative elements.
- Example: Chlorine gains 1 electron: Cl + e⁻ → Cl⁻ (anion).
- The cation (positive ion) and anion (negative ion) attract each other because of opposite charges, forming a chemical bond called an electrovalent (ionic) bond.
- Electrovalent (ionic) compounds are formed when electrons are transferred from one atom to another.
- Electrovalency is the number of electrons an atom loses or gains to form an electrovalent bond.
Electrovalent (or ionic) compounds : The chemical compounds formed as a result of the transfer of electrons from one atom of an element to one atom of another element are called ionic (or electrovalent) compounds.
Electrovalency : The number of electrons that an atom of an element loses or gains to form a electrovalent bond is called its electrovalency
- Metals in groups 1, 2, and 13 tend to lose electrons, while non-metals in groups 15, 16, and 17 tend to gain electrons, forming ionic bonds.
- Note: Group 1 elements are the most electropositive (metallic), with caesium being the most electropositive as metallic nature increases down the group.
- Group 17 elements are the most electronegative, with fluorine being the most electronegative element.
- Caesium fluoride (CsF) is the most ionic compound because caesium is highly electropositive and fluorine is highly electronegative.
- Bonds between metals and non-metals are always ionic (electrovalent) bonds.
- Why are Ionic Compounds Stable?
- Ionic compounds are made of ions with opposite charges that attract each other strongly.
- There is also a repulsive force between ions with the same charge, but the attractive force between opposite charges is much stronger, making ionic compounds stable.
- Examples of electrovalent (ionic) compounds:

Structures of Some Electrovalent Compounds
1. Sodium Chloride (NaCl):
- Sodium has an electronic configuration of 2,8,1, with 1 extra electron compared to the noble gas neon (2,8).
- Sodium loses 1 electron to become stable like neon:
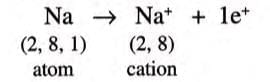
- After losing 1 electron, sodium has 11 protons but 10 electrons, so it gets a +1 charge, becoming a sodium ion (Na⁺).
- Chlorine has an electronic configuration of 2,8,7, which is 1 electron less than the noble gas argon (2,8,8).
- Chlorine gains 1 electron to become stable like argon:
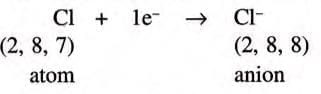
- After gaining 1 electron, chlorine has 17 protons but 18 electrons, so it gets a –1 charge, becoming a chloride ion (Cl⁻).
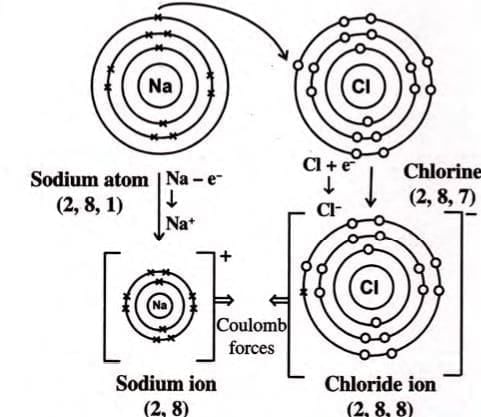
- The Na⁺ and Cl⁻ ions attract each other due to opposite charges, forming the ionic compound NaCl.
Electron dot symbol (Lewis symbol): The electron dot symbol for an atom consists of the symbol of the element surrounded by dots representing the outermost shell electrons. The paired electrons are represented by a pair of dots, whereas the unpaired electron in the outermost orbit is represented by a single dot.E
xample: Electron dot symbol of Hydrogen is H·
and of Oxygen is :O:
Symbols other than dots, such as circles and crosses can be used to distinguish between the electrons of different atoms in a molecule.
for example:
Ammonia (NH₃) can be represented as
2. Magnesium Chloride (MgCl₂):
- Magnesium (atomic number 12) has 2 valence electrons (2,8,2), and chlorine (atomic number 17) has 7 valence electrons (2,8,7).
- Magnesium loses 2 electrons to get a stable configuration of 8 electrons:
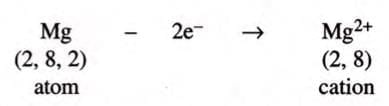
- Each chlorine atom can accept only 1 electron to become stable:
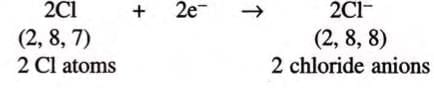
- So, 1 magnesium atom combines with 2 chlorine atoms to form MgCl₂
3. Calcium Oxide (CaO):
- Calcium (atomic number 20) has 2 valence electrons (2,8,8,2), and oxygen has 6 valence electrons (2,6).
- Calcium loses 2 electrons to become stable:
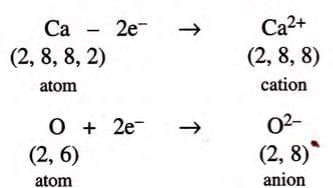
- Oxygen gains 2 electrons to get 8 electrons in its outer shell: O (2,6) + 2 e⁻ → O²⁻ (2,8) (anion).
- 1 calcium atom gives 2 electrons to 1 oxygen atom, so the formula is CaO (not Ca₂O₂).
- Electron dot structure of
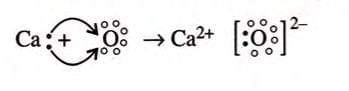
- In electrovalent bonds, electrons are transferred, and this involves a redox process:
- Oxidation: When the electropositive atom (metal) loses electrons (e.g., Na → Na⁺ + e⁻).
- Reduction: When the electronegative atom (non-metal) gains electrons (e.g., Cl₂ + 2 e⁻ → 2 Cl⁻).
- Example: Formation of NaCl:
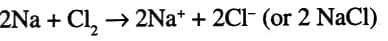 (redox reaction).
(redox reaction).
Half reactions: 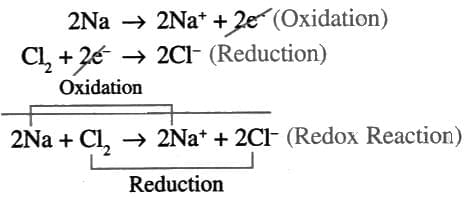
- Oxidation and reduction always happen together because the electrons lost by one atom are gained by another.
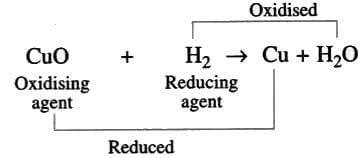
- Example: Hydrogen reduces Cu(II) oxide to copper:
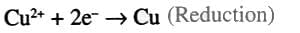 (
( - An oxidizing agent accepts electrons, and a reducing agent donates electrons.
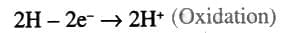
Covalent (Molecular) Bond
- A covalent bond is formed when two atoms share one or more pairs of electrons to become stable.
- Compounds formed by covalent bonds are called covalent compounds, and the molecules formed are called covalent molecules.
- Non-metal atoms usually have 5, 6, or 7 valence electrons (except carbon with 4 and hydrogen with 1).
- These atoms cannot easily lose electrons, so they share electrons with other non-metal atoms to complete their outer shell.
- Each atom gives an equal number of electrons to share.
- When a non-metal combines with another non-metal, they share electrons to form a covalent bond.
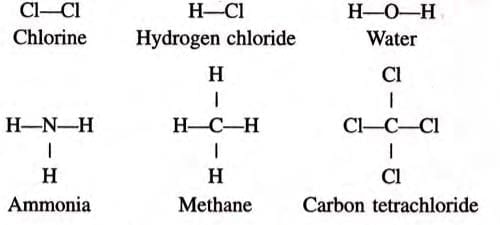
- Example: Hydrogen and chlorine (both non-metals) share electrons to form hydrogen chloride (HCl).
- Covalent bonds can also form between two atoms of the same non-metal.
- Example: Two chlorine atoms share electrons to form a chlorine molecule (Cl₂).
- Types of covalent bonds:
- Single covalent bond: Sharing one pair of electrons (shown as a single line: -).
- Double covalent bond: Sharing two pairs of electrons (shown as a double line: =).
- Triple covalent bond: Sharing three pairs of electrons (shown as a triple line: ≡).
- Examples of single covalent bonds:
- Double bond example: Oxygen molecule (O₂) has a double bond: O=O (two pairs of electrons shared).
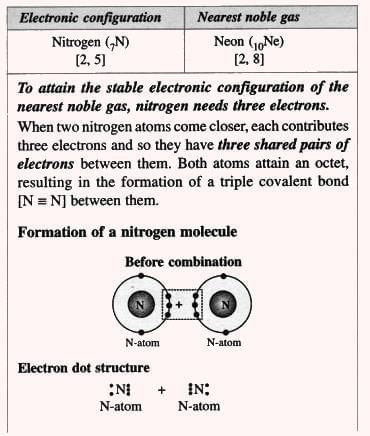
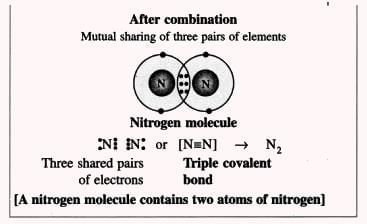
- Triple bond example: Nitrogen molecule (N₂) has a triple bond: N≡N (three pairs of electrons shared).
- Some molecules have both single and double/triple bonds.
- Example: Ethene (C₂H₄) has one double bond and four single bonds: H₂C=CH₂.
Non-polar and Polar Covalent Compounds
- In non-polar covalent compounds, the shared pair of electrons is equally distributed between the two atoms, so there is no charge separation, and the molecule is electrically neutral.
- Examples of non-polar covalent compounds include hydrogen (H₂), chlorine (Cl₂), and oxygen (O₂).
- If the two covalently bonded atoms are identical, the shared electron pair(s) are at an equal distance from both atoms, resulting in equal attraction by the nuclei of the two atoms. This makes the molecule non-polar.
- Examples of non-polar molecules include hydrogen (H₂), chlorine (Cl₂), and oxygen (O₂).
The Covalent Compounds Are Said to Be Polar
- In polar covalent compounds, the shared pair of electrons is not equally distributed between the two atoms, leading to a difference in charge. This results in the development of a small positive and negative charge on the molecule.
- Examples of polar covalent compounds include water (H₂O) and hydrogen chloride (HCl).
Covalent Bond Formed When Both Atoms Have Four or More Electrons in Their Outermost Shells, i.e., Non-metals (Exceptions Are H, Be, B, Al, etc.):
- Non-metals with four or more electrons in their outermost shell form covalent bonds by sharing electrons.
- Exceptions include hydrogen (H), beryllium (Be), boron (B), and aluminum (Al), which can also form covalent bonds despite having fewer valence electrons.
Hydrogen Can Combine with All Non-metals of Group IVA to Form Covalent Bonds
Elements in Group IVA (like carbon, silicon, etc.) can form covalent bonds with hydrogen.
Note:
- As we move across a period in the periodic table, the nature of compounds changes from electrovalent to covalent.
- For example, chlorides, oxides, etc., show a decrease in electrovalent character.
- An example of this trend is seen in the chlorides of the elements in the 3rd period, as shown below:
Some Covalent Molecules and Their Structures
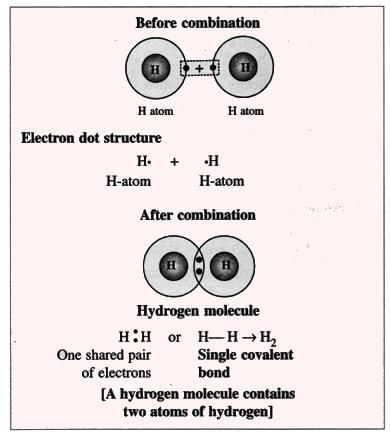
1. Hydrogen Molecule (Non-polar Compound):
- Each hydrogen atom has 1 electron and needs 1 more to get a duplet (2 electrons, like helium).
- Two hydrogen atoms share 1 electron each, forming a pair that both atoms share to make a hydrogen molecule (H₂).
- Formation of a hydrogen molecule
2. Chlorine Molecule (Non-polar Compound):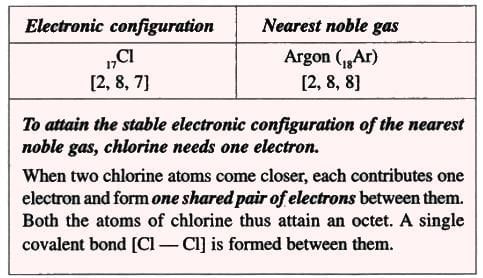
Formation of a chlorine molecule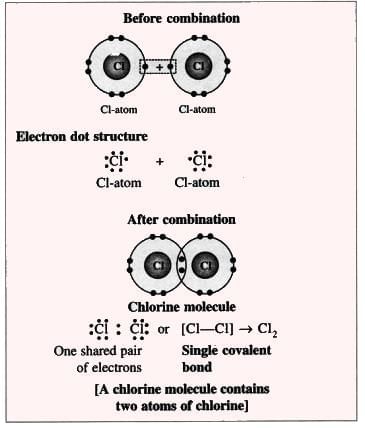
3. Nitrogen Molecule (Non-polar Compound):
4. Hydrogen Chloride (Polar Compound):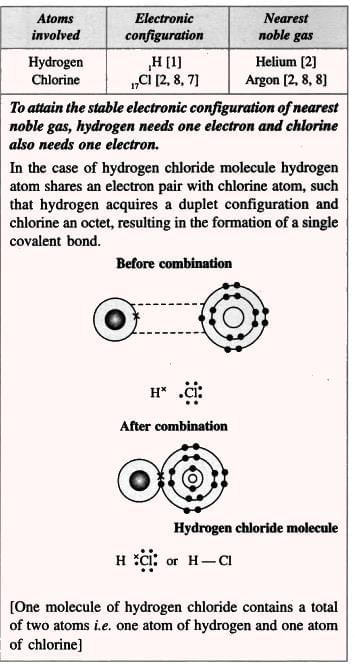
5. Water Molecule (Polar Compound):
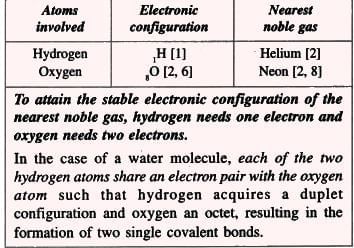
Formation of a water molecule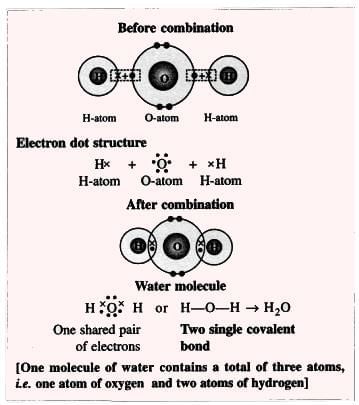
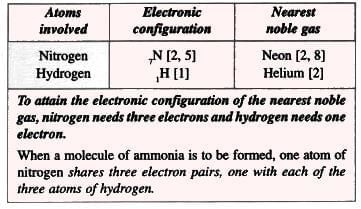
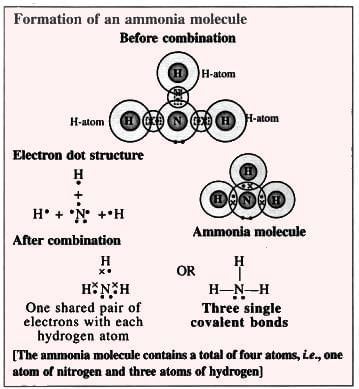
6. Ammonia Molecule (Polar Compound):
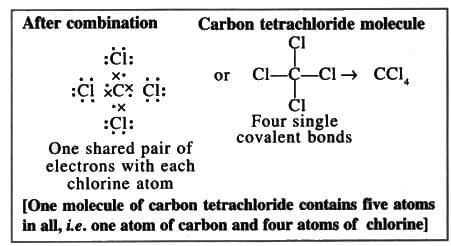
7. Carbon tetrachloride molecule (Non-polar compound)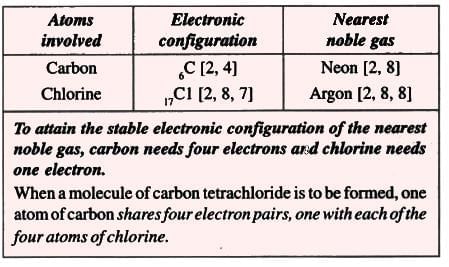
Formation of a carbon tetrachloride molecule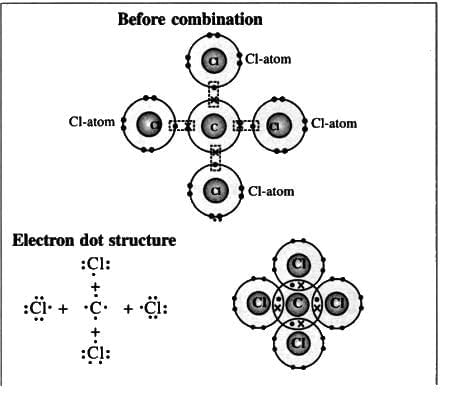
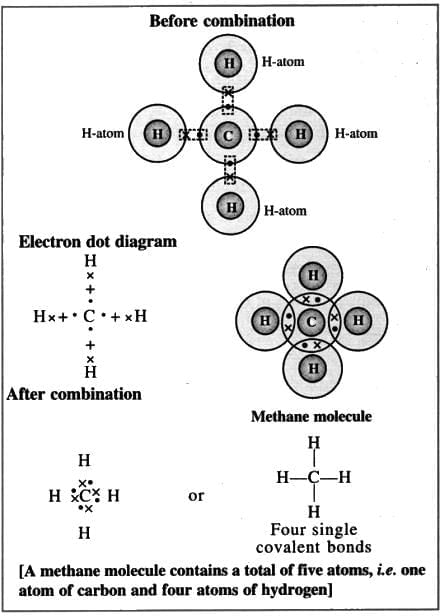
 8. Methane molecule (Non-polar compound)
8. Methane molecule (Non-polar compound)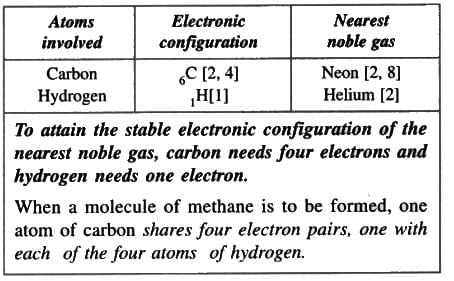
Formation of a methane molecule
Point to Remember
- Greek philosopher Democritus called the parmanu an "atom."
- John Dalton gave the first clear idea about the structure of an atom.
- Gases can conduct electricity at very low pressure (0.01 mm of mercury) and high voltage (10,000 volts), producing rays called cathode rays.
- Cathode rays are made of negatively charged particles called electrons.
- Electrons are present in every atom.
- The mass of an electron is 1/1837 the mass of a hydrogen atom, and its charge is -1.602 × 10⁻¹⁹ coulombs.
- Goldstein discovered positively charged particles using a discharge tube; the nature of these particles depends on the gas used.
- Chadwick discovered the neutron in 1932, a neutral particle with a mass equal to that of a proton.
- Rutherford showed that an atom has a small, positively charged center called the nucleus.
- Electrons, protons, and neutrons are the main particles inside an atom.
- Protons and neutrons are in the nucleus (together called nucleons), and electrons are outside the nucleus in orbits.
- The atomic number (Z) of an element is the number of protons in its nucleus.
- The mass number (A) of an atom is the total number of protons and neutrons in the nucleus.
- Electrons move around the nucleus in fixed circular paths called orbits or energy shells, labeled as 1, 2, 3, 4, or K, L, M, N, etc.
- Electronic configuration is how electrons are arranged in these shells.
- The maximum number of electrons in a shell is given by the formula 2n², where n is the shell number (but the outermost shell cannot have more than 8 electrons).
- The outermost shell is called the valence shell, and the electrons in it are called valence electrons.
- The chemical properties of elements depend on their valence electrons because they take part in chemical reactions.
- Isotopes are atoms of the same element with the same atomic number but different mass numbers (different number of neutrons).
- Isobars are atoms of different elements with the same mass number but different atomic numbers.
- In chemical reactions, atoms become stable by getting the electron arrangement of the nearest noble gas (usually 8 electrons in the outer shell), which is called the octet rule.
- Bonds between metals and non-metals are ionic (electrovalent) bonds.
- A covalent bond is formed when two atoms share one or more pairs of electrons, creating a covalent compound.
|
19 videos|100 docs|23 tests
|
FAQs on Atomic Structure and Chemical Bonding Chapter Notes - Chemistry for SSS 1
| 1. What are subatomic particles, and what role do they play in the structure of atoms? |  |
| 2. How is an element defined in chemistry? |  |
| 3. Who discovered protons, and what was the significance of this discovery? |  |
| 4. What are the main features of Rutherford’s atomic model, and what was its major limitation? |  |
| 5. How does Bohr’s atomic model improve upon Rutherford’s model? |  |