Autoimmunity, Immunodeficiency, Transplantation, and Immunoprophylaxis Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
| Autoimmunity |

|
| Immunodeficiency Disorders |

|
| Transplant Immunology |

|
| Immunoprophylaxis |

|
Autoimmunity
Autoimmunity happens when the body's own immune T-cells or antibodies wrongly attack its own self-antigens, causing damage to their structure or function.
Immunological Tolerance
- State where an individual cannot mount an immune response against their own tissue antigens.
- Mediated by two mechanisms: central tolerance and peripheral tolerance.
Central Tolerance
Eliminates self-reactive T and B lymphocytes during their maturation in central lymphoid organs.
- In thymus: Negative selection removes self-reactive T-cells.
- In bone marrow: Negative selection and receptor editing remove self-reactive B-cells.
- Example: In the thymus, a T-cell recognizing a self-antigen like insulin is deleted to prevent it from attacking pancreatic cells, which could lead to type 1 diabetes.
Peripheral Tolerance
Backup mechanisms in peripheral tissues to control self-reactive T-cells escaping central tolerance.
- Ignorance: Self-reactive T-cells fail to encounter self-antigens, remaining inactive.
- Anergy: T-cells become unresponsive due to CTLA-4 on T-cells binding B7 on antigen-presenting cells (APCs), blocking co-stimulatory signals.
- Phenotypic Skewing: Self-reactive T-cells produce non-harmful cytokines upon stimulation.
- Apoptosis: Self-reactive T-cells undergo activation-induced cell death (AICD).
- Regulatory T-cells (Treg cells): Suppress self-reactive T-cells.
- Dendritic Cells (DCs): Immature or tolerogenic DCs capturing self-antigens downregulate co-stimulatory molecules (e.g., CD40, B7) or induce Treg cells.
- Sequestration:Self-antigens in immunologically privileged sites (e.g., cornea, testes, brain) avoid immune detection.
- Example: In the eye, corneal proteins are sequestered, preventing immune attack, which explains why corneal transplants have lower rejection rates.
Mechanisms of Autoimmunity
- CTLA-4 mediated T-cell anergy: This phenomenon is observed in conditions like multiple sclerosis, rheumatoid arthritis, and psoriasis.
- Failure of activation-induced cell death (AICD): This issue is commonly seen in systemic lupus erythematosus (SLE).
- Decrease in Treg cells: A reduction in regulatory T cells can impact immune function.
- Assisting T-cells: T-cells can help activate self-reactive B-cells, which may contribute to autoimmune responses.
- Release of hidden antigens: Damage to certain organs can lead to the release of antigens, such as those found in spermatozoa and ocular antigens.
- Molecular mimicry: This occurs in conditions like post-streptococcal acute rheumatic fever and glomerulonephritis, where the immune system mistakenly targets the body due to similarities with pathogens.
- Polyclonal lymphocyte activation: This is driven by factors such as superantigens, as well as infections like EBV (Epstein-Barr virus) and HIV.
- Exposing hidden self-epitopes: Certain processes can reveal previously concealed parts of our own proteins that the immune system may react to.
- Epitope spreading: This refers to the immune response expanding to target new parts of an antigen over time.
- Bystander activation: This phenomenon occurs when immune cells are activated not because they are directly targeted but due to the overall immune response in the area.
Autoimmune diseases
Single organ or cell type autoimmune diseases
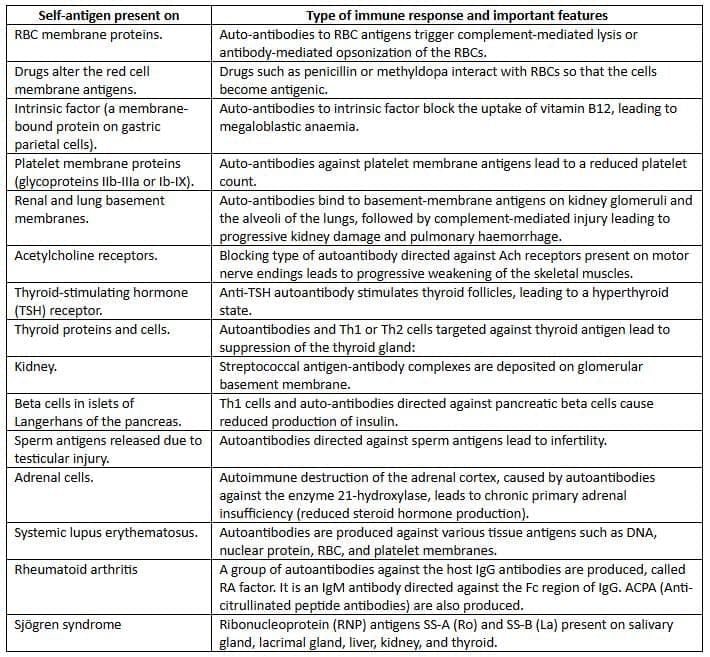
Immunodeficiency Disorders
Immunodeficiency refers to a condition where the body's defense mechanisms are weakened, making it easier for infections and some types of cancer to occur.
This condition is generally divided into two categories: primary and secondary.
- Primary immunodeficiency diseases are caused by inherited problems that affect how the immune system develops.
- Secondary immunodeficiency diseases occur as a result of other health issues that disrupt the normal functioning of the immune system. These can include:
- Infections
- Malnutrition
- Aging
- Immunosuppression
- Autoimmunity
- Chemotherapy
- Secondary immunodeficiency diseases are more common than primary immunodeficiency diseases.
Primary Immunodeficiency Diseases
Humoral Immunodeficiency (B-cell Defects)
- Bruton Disease (X-linked Agammaglobulinemia):
- Failure of pre-B cells to differentiate into immature B-cells due to absent Bruton’s tyrosine kinase.
- Total absence of B-cells, plasma cells, and all immunoglobulin classes.
- B-cell maturation stops at pre-B cell stage after heavy-chain synthesis, without light-chain formation.
- X-linked, primarily in males, onset after 6 months.
- Recurrent bacterial, viral (enteroviruses), and parasitic (Giardia lamblia) infections.
- Autoimmune diseases (e.g., SLE, dermatomyositis) in up to 20% of cases.
- Common Variable Immunodeficiency:
- Defective B-cell differentiation, leading to low immunoglobulin levels.
- Isolated IgA Deficiency:
- Most common primary immunodeficiency (1 in 700 white individuals).
- Defective mucosal immunity causes recurrent sinopulmonary infections and diarrhea.
- Significant association with autoimmune diseases.
- Pathogenesis: Block in IgA-secreting B-cell differentiation to plasma cells, due to altered T-cell cytokine production (e.g., TGF-β, IL-5) or intrinsic B-cell defect.
- Normal or excess levels of other immunoglobulins.
- Hyper-IgM Syndrome:
- X-linked disorder due to defective isotype class switching from mutations in CD40 or CD40 ligand.
- High IgM levels, lack of other antibody classes.
- Leads to secondary infections and autoimmune diseases.
- Transient Hypogammaglobulinemia of Infancy:
- Delayed IgG synthesis (sometimes IgA or IgM), starting normally by 2 months.
- Defective opsonization or complement activation causes recurrent otitis media and respiratory infections.
- Self-limiting, resolves by 6 months.
- Normal antibody response to vaccines.
Cellular Immunodeficiencies (T-cell Defects)
- DiGeorge Syndrome (Thymic Hypoplasia):
- Congenital thymic development defect impairs T-cell maturation.
- Rare autosomal recessive disorder (1 in 100,000 live births), also called velocardiofacial syndrome.
- Pathogenesis: 80% cases involve chromosome 22q11 deletion, causing third and fourth pharyngeal pouch malformation in embryonic life.
Disorders of Phagocytosis
- Impairments in phagocyte function increase infection susceptibility.
Disorders of Complement
- Complement component deficiencies.
- Complement regulatory protein deficiencies.
Risk of Secondary Infections in Various Immunodeficiencies
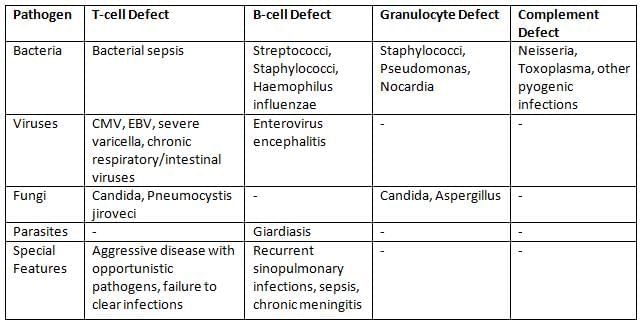
Transplant Immunology
Based on genetic relationship between donor and recipient:
- Autograft: Self-tissue transferred within the same individual (e.g., skin grafts).
- Isograft/Syngeneic Graft: Tissue transferred between genetically identical individuals (e.g., monozygotic twins).
- Allograft: Tissue transferred between genetically non-identical members of the same species.
- Xenograft: Tissue transferred between different species (e.g., baboon heart).
Types of Graft Rejection
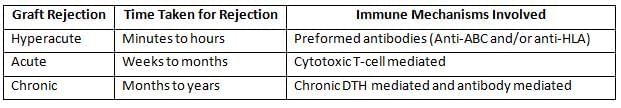
Mechanism of Graft Rejection
Two stages:- Sensitization Phase: Alloantigens (mainly graft MHC molecules) presented to recipient’s T-cells via:
- Direct Pathway: Graft APCs present MHC to recipient’s T-cells, leading to acute rejection by cytotoxic T-cells.
- Indirect Pathway: Recipient APCs process graft antigens, contributing to chronic rejection.
- Effector Phase: Activated T-cells and antibodies attack the graft.
- Example: In a kidney transplant, if donor MHC molecules are presented directly to recipient T-cells, cytotoxic T-cells may attack the graft within weeks, causing acute rejection.
Laboratory Tests to Determine Histocompatibility
- ABO Blood Group Compatibility: Tested by blood grouping and cross-matching.
- HLA Typing: Matches donor and recipient leukocyte antigens or genes.
- Phenotypic Methods: Microcytotoxicity (serology), mixed lymphocyte reaction (tissue typing).
- Genotypic Methods: PCR for HLA genes, PCR-RFLP, VNTR typing, STR typing, DNA sequence-based typing, karyosome analysis.
Graft-Versus-Host Reaction (GVH)
Graft’s immune cells attack recipient’s tissues, opposite to typical graft rejection.
- Occurs when:
- Graft contains immunocompetent T-cells (e.g., bone marrow, stem cell transplants).
- Recipient has transplantation antigens absent in the graft.
- Recipient is immunologically suppressed, unable to counter graft’s attack.
- Forms:
- Acute GVH: Within 100 days, damages liver (hepatomegaly), skin (rash), mucosa, intestines (diarrhea).
- Chronic GVH: After 100 days, affects liver, skin, mucosa, intestines, plus connective tissues and exocrine glands.
- Experimental model: Runt disease in mice.
- Treatment: Glucocorticoids.
- Example: In bone marrow transplant, donor T-cells recognizing recipient’s skin antigens may cause rash and diarrhea, indicating acute GVH.
Tumor Antigens
Tumor-Specific Transplantation Antigen (TSTA):
- Present only on tumor cells, absent in normal cells.
- Induced by chemical/physical or viral carcinogens.
- Chemically/physically induced tumors have unique TSTA.
- Virus-induced tumors share virus-specific TSTA.
Tumor-Associated Antigens (TATAs):
- Expressed on tumor and normal cells (at low levels).
- Examples: Oncofoetal antigens, carcinoembryonic antigen (CEA), carbohydrate antigens (CA 125, CA 19-9), prostate-specific antigen, β2 macroglobulin.
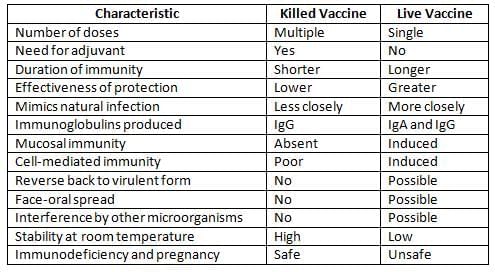
Immunoprophylaxis
Characteristics of Killed and Live Vaccines
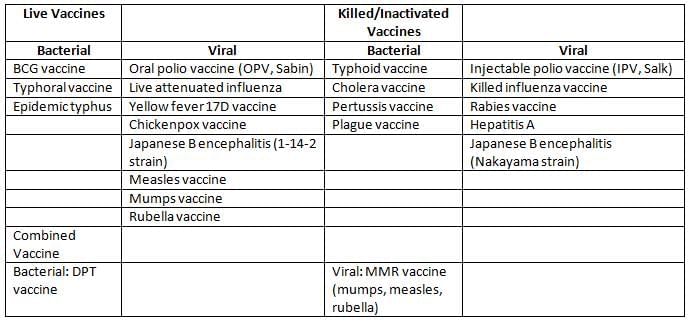
Examples of Vaccines
National Immunization Schedule (NIS) for Infants, Children, and Pregnant Women (India)
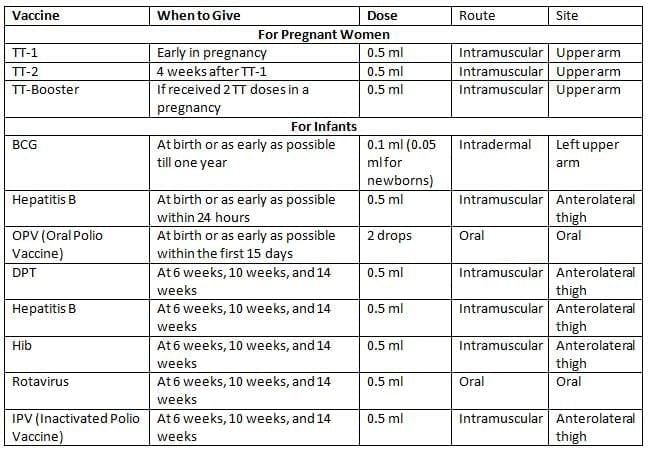
- TT-2 or Booster doses should be given before 36 weeks of pregnancy. However, these should still be administered even if more than 36 weeks have passed. TT should be given to a woman in labor if she has not received it before.
- SA 14-14-2 Vaccine is available in certain areas of Uttar Pradesh, Karnataka, West Bengal, and Assam where the disease is common.
- The 2nd to 9th doses of Vitamin A can be given to children aged 1 to 5 years.
Immunoglobulin Preparations
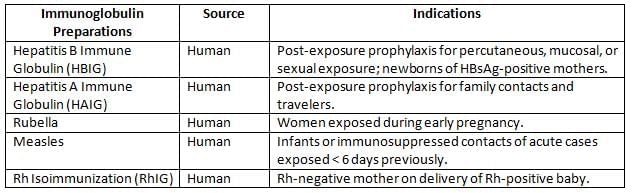
Example: A newborn of an HBsAg-positive mother receives HBIG within 12 hours of birth to prevent hepatitis B transmission, alongside the hepatitis B vaccine.
|
75 docs|5 tests
|
FAQs on Autoimmunity, Immunodeficiency, Transplantation, and Immunoprophylaxis Chapter Notes - Microbiology - NEET PG
| 1. What is immunological tolerance and why is it important in preventing autoimmune diseases? |  |
| 2. What are the primary mechanisms involved in the development of autoimmunity? |  |
| 3. How does graft rejection occur and what mechanisms are involved in this process? |  |
| 4. What laboratory tests are commonly used to determine histocompatibility for organ transplantation? |  |
| 5. What is a graft-versus-host reaction and in what situations does it typically occur? |  |



















