Bacterial Genetics and Antimicrobial Resistance Chapter Notes | Preparation for EmSAT Grade 10 PDF Download
Bacterial Genetics
Bacterial genetics involves studying how bacteria pass on and change their traits. All hereditary information in bacteria is stored in their DNA, which is located in chromosomes and in additional DNA called plasmids.
Plasmids
Plasmids are circular DNA structures that are double-stranded and exist freely in the bacterial cytoplasm. They are not essential for the bacteria's survival, as bacteria can gain or lose plasmids at any point in their life cycle. Plasmids can replicate independently and can be found in some yeasts as well.
- Numbers: Bacteria may have one or many plasmids, with some types containing more than 40 plasmids in a single cell.
- Episome:. plasmid that integrates into the chromosomal DNA of bacteria is called an episome.
- Curing: The process of removing plasmids from bacteria is referred to as curing.
Classification of Plasmids
- Based on Ability to Perform Conjugation:
- Conjugative Plasmids or Self-Transmissible Plasmids: These plasmids have the ability to transfer themselves from one bacterial cell to another through the process of conjugation. They carry the necessary genes for forming the conjugation tube and facilitating the transfer of genetic material.
- Non-Conjugative Plasmids or Non-Transmissible Plasmids: These plasmids do not possess the genes required for self-transfer. They cannot move from one cell to another on their own and typically rely on other mechanisms for propagation within a bacterial population.
- Based on Compatibility:
- Compatible Plasmids: These plasmids can coexist within a single bacterial cell without interfering with each other. They have distinct replication origins that allow them to replicate simultaneously in the same host.
- Incompatible Plasmids: Incompatible plasmids cannot exist together in the same bacterial cell. They often have similar replication origins, leading to competition for replication machinery, which results in the loss of one of the plasmids from the cell.
- Based on Function:
- Fertility or F-Plasmids: These plasmids carry genes that code for sex pili, which are appendages that facilitate the formation of a conjugation tube. This tube is essential for the transfer of genetic material between bacterial cells during conjugation.
- Resistance (R) Plasmids:. plasmids contain genes that confer resistance to various antibiotics. They play a crucial role in the survival of bacteria in environments containing antimicrobial agents by providing the means to neutralize or evade the effects of these drugs.
- Col Plasmids: Col plasmids carry genes that code for bacteriocins, which are antimicrobial substances produced by bacteria to inhibit the growth of closely related or competing bacterial strains. These plasmids contribute to the competitive advantage of the host bacteria in their natural environments.
- Virulence Plasmids: These plasmids encode virulence factors such as toxins and adhesins, which enhance the pathogenicity of the bacteria. Toxins can cause damage to host tissues, while adhesins facilitate the attachment of bacteria to host surfaces, promoting infection.
- Metabolic Plasmids: Metabolic plasmids are involved in various metabolic activities within the host bacterium. They may carry genes that enable the degradation of specific substrates, utilization of unusual carbon sources, or other metabolic pathways that provide the host with additional capabilities to exploit environmental resources.
- Plasmid as Vector:
- Conjugative Plasmids as Vectors: Due to their natural ability to transfer DNA from one bacterial cell to another, conjugative plasmids are utilized as important vectors in genetic engineering. These plasmids can be engineered to carry specific genes of interest and facilitate their introduction into target bacterial cells.
- Recombinant DNA Technology: Conjugative plasmids have specific sites where foreign genes can be inserted using recombinant DNA technology. This involves cutting the plasmid at precise locations, inserting the gene of interest, and ligating the DNA fragments to create a recombinant plasmid.
- Applications: Engineered conjugative plasmids can be used for various applications such as protein production, where the plasmid carries the gene for a desired protein and directs its expression in the host bacterium. They are also used in gene therapy, where therapeutic genes are delivered into target cells to correct genetic disorders or enhance specific functions.
Horizontal Gene Transfer in Bacteria
Gene transfer in bacteria can be broadly divided into:
- Vertical gene transfer: transmission of genes from parents to offspring.
- Horizontal gene transfer: transmission of genes from one bacterium to another. This occurs through:
Transformation
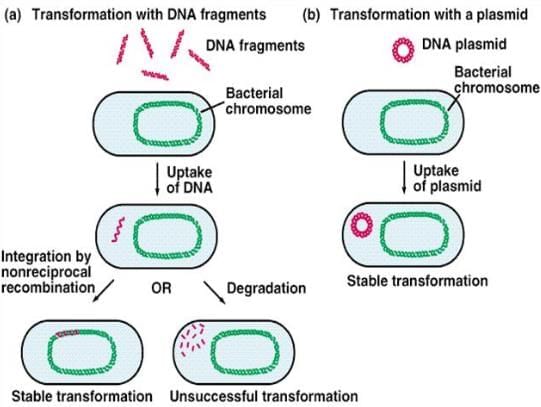
Transformation is a process by which a bacterial cell takes up free or naked DNA fragments from its environment and incorporates them into its own chromosome, making these genes heritable. This phenomenon has been observed in specific bacteria, including:
- Streptococcus pneumoniae
- Bacillus
- Haemophilus
- Neisseria
- Acinetobacter
- Pseudomonas
The Griffith experiment conducted in 1928, provided significant evidence for the occurrence of transformation in bacteria. It demonstrated that non-virulent strains of Streptococcus pneumoniae could acquire virulence when exposed to heat-killed virulent strains.
Transduction
Transduction is a method of genetic transfer in bacteria where a bacteriophage, a type of virus that infects bacteria, transfers DNA from one bacterium to another.
There are two main types of transduction:
- Generalized Transduction: In this type, any part of the donor bacterium's genome can be transferred to the recipient bacterium. This occurs when the bacteriophage accidentally incorporates fragments of the bacterial DNA into its own viral DNA and then injects it into another bacterium.
- Specialized Transduction: This type involves the transfer of a specific portion of the bacterial chromosome that is located near the phage DNA. This happens when the bacteriophage excises its DNA from the bacterial chromosome and takes along a specific segment of the bacterial DNA.
Role of Transduction:
- Transduction plays a significant role in the transfer of various genetic materials, including plasmids, between bacteria. It is a crucial mechanism for the spread of drug resistance genes, such as those that confer resistance to penicillin in Staphylococcus species.
- Additionally, transduction is utilized in genetic engineering to develop treatments for certain inherited metabolic disorders.
- For example, scientists can use modified bacteriophages to deliver therapeutic genes into patients' cells to correct metabolic deficiencies.
- In the context of genetic engineering, transduction is a valuable tool for transferring specific genes or genetic elements into target organisms. This technique can be employed to introduce beneficial traits, such as disease resistance or improved metabolic capabilities, into bacterial strains used in industrial or therapeutic applications.
- By harnessing the natural ability of bacteriophages to transfer genetic material, researchers can develop innovative solutions for various genetic disorders and enhance the capabilities of microbial strains for biotechnological purposes.
Lysogenic Conversion
- In the temperate or lysogenic phase, phage DNA integrates into the bacterial chromosome, forming a prophage.
- The prophage imparts new characteristics to the bacterial daughter cells.
- It enables bacteria to produce toxins, with phage DNA coding for various toxins known as ABCDE:
- A and C of Streptococcus pyrogenic exotoxin (SPE)
- Botulinum toxin C and D
- Cholera toxin
- Diphtheria toxin E. coli (Verocytotoxin)
Conjugation
Conjugation is a process where genetic material is transferred from one bacterium to another through mating and the formation of a conjugation tube. This process was first discovered by Lederberg and Tatum.
- F+ × F– Mating: In this type of conjugation, when an F+ cell (carrying a plasmid known as the F factor or fertility factor) approaches an F– cell (which lacks the F factor), the F factor facilitates the formation of a conjugation tube. This tube enables the transfer of the F factor to the F– cell, transforming it into an F+ cell.
- HFR Conjugation: In this process, the F factor, existing as a plasmid, can integrate into the bacterial chromosome and function like an episome.
- Donor cells with integrated F factors are known as Hfr cells (high frequency of recombination) because they can transfer chromosomal DNA to recipient cells more frequently than F+ cells.
- During conjugation between an Hfr cell and an F– cell, only a portion of chromosomal genes and part of the F factor may be transferred, preventing the F– recipient from becoming an F+ cell.
- F′ Conjugation:The transition from an F+ cell to an Hfr cell can be reversed.
- When the F factor reverts from its integrated state to a free state, it may carry some adjacent chromosomal DNA with it. This modified F factor is called the F′ factor (F prime factor).
- When an F′ cell conjugates with an F– recipient, it transfers both the F factor and the host DNA it carries, resulting in the recipient becoming an F′ cell. This process is referred to as sexduction.
- Conjugation plays a crucial role in the transfer of plasmids that encode resistance to antibacterial drugs, known as resistance transfer factors (RTFs), and in the production of bacteriocins, such as Colicinogenic or Col factors.
- The R factor(resistance factor) is a type of plasmid composed of two parts:
- Resistance transfer factor (RTF): This component is responsible for the conjugational transfer of the plasmid, similar to the F factor.
- Resistance determinant (r): This part codes for resistance to a specific drug. An R factor can possess multiple r determinants, providing resistance to various drugs.
Antimicrobial Resistance
Antimicrobial resistance can be classified into two types: intrinsic and acquired.
Intrinsic Resistance
Intrinsic resistance refers to the natural ability of certain bacteria to withstand specific groups of antibiotics. This type of resistance is inherent to the bacterial species and is not acquired through genetic changes. For example:
- Anaerobic bacteria have intrinsic resistance to aminoglycosides.
- Aerobic bacteria are intrinsically resistant to metronidazole.
- Gram-negative bacteria exhibit intrinsic resistance to vancomycin.
- Klebsiella species are intrinsically resistant to ampicillin, sulfonamides, trimethoprim, tetracycline, and chloramphenicol.
- Enterococci have intrinsic resistance to aminoglycosides and all cephalosporins.
Acquired Resistance
Acquired resistance occurs when bacteria develop the ability to resist antibiotics by acquiring genes that confer drug resistance. This can happen through various mechanisms, including:
- Mutation: Bacteria can develop resistance to antibiotics through spontaneous mutations in their genetic material. These mutations can alter the target site of the antibiotic, making it ineffective.
- Transferable Drug Resistance: Resistance can be transferred between bacteria through horizontal gene transfer mechanisms such as conjugation, transduction, or transformation. This allows bacteria to acquire resistance genes from other resistant strains.
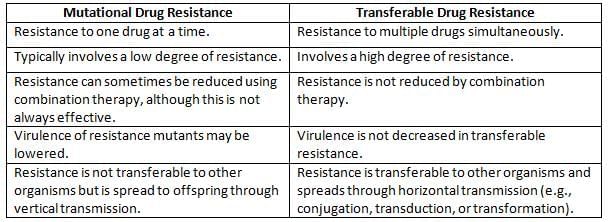
Characteristics of Mutational Drug Resistance vs. Transferable Drug Resistance
Mechanism of Antimicrobial Resistance
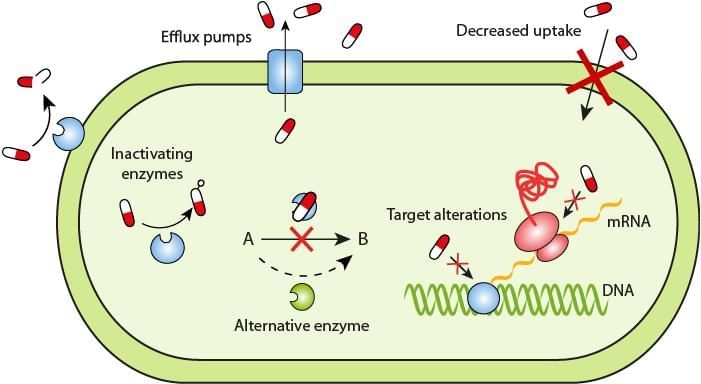
Bacteria can develop antimicrobial resistance through various mechanisms:
1. Decreased permeability across the cell wall: Some bacteria alter their cell membrane porin channels, preventing antimicrobials from entering. This is seen in many gram-negative bacteria like Pseudomonas, Enterobacter, and Klebsiella species against drugs such as imipenem, aminoglycosides, and quinolones.
2. Efflux pumps: Certain bacteria have pumps that quickly expel drugs from the cell, reducing drug levels inside. This mechanism has been noted in:
- Escherichia coli and other Enterobacteriaceae against tetracyclines and chloramphenicol.
- Staphylococci against macrolides and streptogramins.
- Staphylococcus aureus and Streptococcus pneumoniae against fluoroquinolones.
3. Modification of antimicrobial target sites: This occurs in:
- MRSA (Methicillin-resistant Staphylococcus aureus).
- VRE (Vancomycin-resistant Enterococci).
- Resistance to streptomycin in Mycobacterium tuberculosis. due to changes in ribosomal proteins or 16S rRNA.
- Resistance to rifampicin in Mycobacterium tuberculosis. due to mutations in RNA polymerase.
- Quinolone resistance (seen in S. aureus and S. pneumoniae): due to mutations in the DNA gyrase enzyme.
4. Enzymatic inactivation: Some bacteria can inactivate antimicrobial agents by producing various enzymes, such as:
- Aminoglycoside-modifying enzymes (acetyltransferases, adenyltransferases, phosphotransferases), produced by both gram-negative and gram-positive bacteria, which modify aminoglycosides.
- Chloramphenicol acetyl transferase: It is produced by members of Enterobacteriaceae.
- β-lactamase enzyme production.
Beta-Lactamase Enzymes
β-lactamase enzymes can break down the β-lactam rings (the active site) of β-lactam antibiotics, making them ineffective. This is observed in both gram-positive and gram-negative bacteria.
These enzymes are often plasmid-coded and can be transferred between bacteria mainly through conjugation (except in Staphylococcus aureus, where they are transferred by transduction).
Beta-lactamases can be classified in two main ways:
- Ambler’s classification (structural or molecular classification)—See table.
- Bush-Jacoby-Medeiros classification (functional or phenotypic)—an advanced and complex classification.
Ambler classification of beta-lactamases
- Class A - ESBL (Extended-spectrum β-lactamases):
- Organisms with ESBL enzymes are resistant to all penicillins and 1st, 2nd, and 3rd generation cephalosporins and monobactams, but remain sensitive to carbapenems and cephamycins.
- Resistance can be overcome by a combination of β-lactam and β-lactamase inhibitors (e.g., sulbactam or clavulanic acid).
- Detected by the combination disk test (Ceftazidime and Ceftazidime + clavulanic acid) and the three-dimensional test (best method).
- Class B - MBL (Metallo beta-lactamase):
- These organisms are resistant to all antibiotics that AmpC beta-lactamase producers are resistant to, as well as to carbapenems.
- Resistance cannot be overcome by β-lactam and β-lactamase inhibitor combinations.
- Detected by the EDTA disk synergy test and modified Hodge test.
- Class C - AmpC beta-lactamase:
- These organisms are resistant to all antibiotics that ESBL producers are resistant to, plus they are resistant to cephamycins (e.g., cefoxitin and cefotetan), but sensitive to carbapenems.
- Detected by the AmpC disk test using cefoxitin disk.
- Class D - Oxacillinase:
- Resistance can be overcome by β-lactam and β-lactamase inhibitor combinations.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing methods consist of the following:
- Disk diffusion methods: Kirby-Bauer disk diffusion method and Stokes disk diffusion method:
- Mueller-Hinton agar (MHA) is the standard medium used for testing.
- The lawn culture method is used to inoculate the organism onto MHA.
- Control strains: ATCC (American Type Culture Collection) strains are employed.
- Reporting follows CLSI (Clinical and Laboratory Standards Institute) guidelines.
- Dilution tests: Broth dilution method and agar dilution method:
- The antimicrobial agent is serially diluted, and each dilution is tested with the organism.
- MIC (minimum inhibitory concentration) is the lowest concentration that prevents visible growth.
- Epsilometer or E-test: A quantitative method that detects MIC using both dilution and diffusion principles.
- Automated methods such as VITEK 2, Phoenix System, and MicroScan WalkAway system.
- Molecular methods PCR is used to detect drug-resistant genes, e.g., MecA gene for MRSA.
|
29 videos|254 docs|56 tests
|
FAQs on Bacterial Genetics and Antimicrobial Resistance Chapter Notes - Preparation for EmSAT Grade 10
| 1. What is the significance of antimicrobial susceptibility testing in clinical practice? |  |
| 2. How do bacteria develop antimicrobial resistance? |  |
| 3. What are the common methods for performing antimicrobial susceptibility testing? |  |
| 4. What role do plasmids play in bacterial resistance to antibiotics? |  |
| 5. Why is monitoring antimicrobial resistance trends important in public health? |  |
















