Chemical Reactions Chapter Notes | Chemistry Class 8 ICSE PDF Download
Introduction
In this chapter, we will learn about chemical reactions, which are processes where substances change to form new substances. We will study different types of reactions like combination, decomposition, displacement, double displacement, precipitation, and neutralization. We will also explore examples, activities, and facts to understand how these reactions happen in our daily lives, such as burning of magnesium or digestion of food in our body.
Chemical Reactions
A chemical reaction is a process where one or more substances change into new substances. It happens when the tiny particles, called atoms, of the starting substances break apart and join in new ways to form different substances.
The starting substances are called reactants, and the new substances made are called products. During a chemical reaction, you might see things like a color change, a gas being made, or a solid forming.
These reactions need certain conditions like heat, light, or mixing the substances properly to happen. In this chapter, we will learn how chemical reactions work, the different types, and what helps them happen.
Example: Sodium carbonate reacts with hydrochloric acid to form sodium chloride, water, and carbon dioxide.
The chemical equation can be represented as shown:
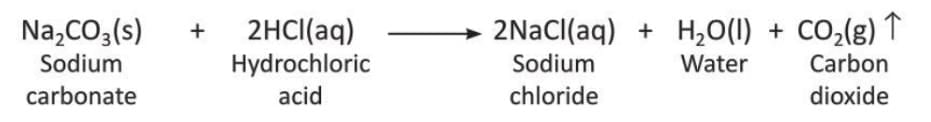
Sodium carbonate and hydrochloric acid are reactants, and sodium chloride, water, and carbon dioxide are products in the above chemical reaction.
Characteristics of a Chemical Reaction
1. Evolution of Gas: Some chemical reactions make gas as a product. We can see the gas as bubbles if the reaction happens in a liquid. This bubbling is called effervescence. The gas that comes out is shown by an upward arrow (↑) in the reaction equation.
Examples:
- When we add zinc granules to dilute sulfuric acid, hydrogen gas comes out. We can see bubbles near the mouth of the test tube because of the gas. If we bring a burning matchstick near the test tube, the gas burns with a pop sound.

- When we heat solid sodium nitrate, oxygen gas comes out. If we bring a glowing splinter near the test tube, the splinter bursts into flame because of the oxygen gas.

- When we heat solid calcium carbonate with lime water, carbon dioxide gas comes out. If this gas is passed through lime water, the lime water turns milky.

Effervescence: The bubbling of a solution due to the escape of gas is called effervescence.
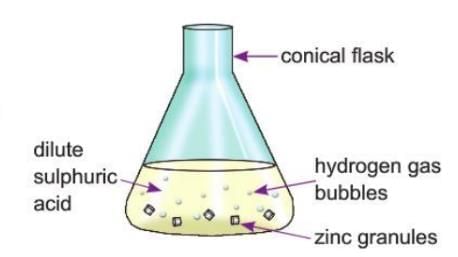
Activity 6.1
Aim: The goal is to observe the release of hydrogen gas when zinc granules react with dilute sulfuric acid.
Materials: You need zinc granules, a conical flask, and dilute sulfuric acid.
Procedure:
- Place zinc granules in a conical flask.
- Add dilute sulfuric acid to the flask, covering the zinc granules.
Observation: When the acid is added, you’ll see bubbles forming around the zinc granules. These bubbles are hydrogen gas being produced.
Conclusion: The reaction between zinc and dilute sulfuric acid produces hydrogen gas. The chemical equation for this reaction is:
Zn + H2SO4 → ZnSO4 + H2
Zinc reacts with sulfuric acid to form zinc sulfate (a salt) and hydrogen gas, which is observed as bubbles. This is a classic example of a metal-acid reaction where a metal displaces hydrogen from the acid, releasing hydrogen gas.
2. Change of Colour: Some chemical reactions show a change in color. The starting substances (reactants) and the new substances (products) have different colors.
Examples:
- When we heat solid lead nitrate, it gives reddish-brown nitrogen dioxide gas and yellow-colored lead monoxide. Oxygen gas is also formed in this reaction.

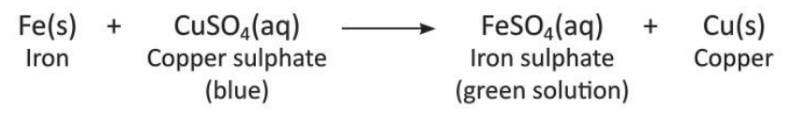
- When we drop an iron nail in blue-colored copper sulphate solution, the blue color fades and turns light green. A reddish-brown layer of copper forms on the iron nail.
- When we heat green-colored copper(II) carbonate strongly, it breaks down to form black-colored copper(II) oxide. Carbon dioxide gas is also released.

Activity 6.2
Aim: To observe colour change during a chemical reaction
Materials: A beaker, copper sulphate, an iron nail, some water
Procedure:
- Take some water in a beaker. Add some copper sulphate in it. You will get a blue coloured solution.
- Drop an iron nail into it. Leave it for a few hours.
Observation: The blue colour changes to green colour.
Conclusion: This shows that a new substance has formed. This confirms that chemical reactions are accompanied by change in colour.
3. Formation of Precipitate: Some chemical reactions form an insoluble solid called a precipitate. The precipitate is shown by a downward arrow (↓) in the reaction equation.
Examples:
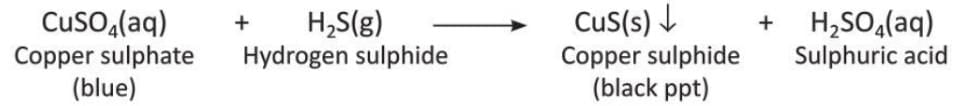
- When hydrogen sulphide gas is passed through a blue-colored copper sulphate solution, a black precipitate of copper sulphide forms.
- When ammonium hydroxide and iron sulphate solutions are mixed, a dirty-green precipitate of iron hydroxide forms.

- When sodium chloride and silver nitrate solutions are mixed, a white precipitate of silver chloride forms.

Precipitate: A solid substance separated from a solution is called a precipitate.
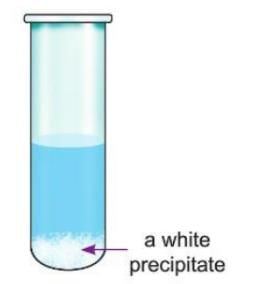
Activity 6.3
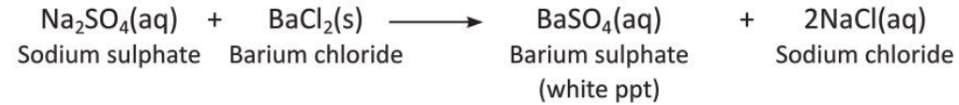
Aim: To show that a precipitate forms during a chemical reaction using sodium sulphate and barium chloride solutions.
Materials required: Sodium sulphate solution, barium chloride solution, two test tubes.
Procedure:
- Take sodium sulphate solution in one test tube.
- Take barium chloride solution in another test tube.
- Mix the two solutions in one test tube.
Observation: When sodium sulphate and barium chloride are mixed, a white precipitate of barium sulphate is formed.
Conclusion: Mixing sodium sulphate and barium chloride solutions forms a white precipitate of barium sulphate.
4. Change of State: In some chemical reactions, the physical state of substances changes. The states can be solid (s), liquid (l), or gas (g), and they are written in brackets in equations.
Examples:
- Hydrogen gas and oxygen gas combine to form water, which is a liquid.

- Ammonia gas and hydrogen chloride gas combine to form solid ammonium chloride.

5. Change in Energy: Some chemical reactions involve energy changes, like absorbing or giving out heat, light, or other energy. Reactions are of two types based on energy: exothermic (gives out energy) and endothermic (absorbs energy).
In an exothermic reaction, heat energy is given out. The chemical reaction in which heat energy is evolved is called an exothermic reaction.
Example:
- When water is added to quicklime, heat energy is released, and calcium hydroxide forms.

- In another exothermic reaction, when carbon burns in oxygen, heat energy and carbon dioxide are produced.

In an endothermic reaction, heat energy is absorbed. The chemical reaction in which heat energy is absorbed is called an endothermic reaction.
Example:
- When carbon and sulphur are heated together, heat energy is absorbed to form carbon disulphide.

- Another endothermic reaction: When nitrogen and oxygen are heated at 3000°C, nitric oxide forms by absorbing heat.

- In another reaction, plants use sunlight to make food in a process called photosynthesis, which is endothermic.

Conditions Necessary for Chemical Reactions
Chemical reactions need certain conditions to happen. Some need heat, light, or electricity, while others need high pressure or a special substance called a catalyst.
1. Close contact
- Reactants must touch each other for a chemical reaction to happen.
- Example: Sodium reacts with water only when they are in contact.

2. Presence of solution
- Some reactions happen faster when reactants are dissolved in a liquid (solution).
- Example: Sodium sulphate and barium chloride react faster when mixed in a solution to form barium sulphate precipitate.
3. Heat energy
- Some reactions need heat to happen.
- Example: Calcium carbonate breaks into calcium oxide and carbon dioxide when heated.

4. Light energy
- Some reactions happen in the presence of light. These are called photochemical reactions.
- Example 1: Photosynthesis in plants uses sunlight to make glucose and oxygen.

- Example 2: Silver chloride breaks down in light to form silver and chlorine gas.

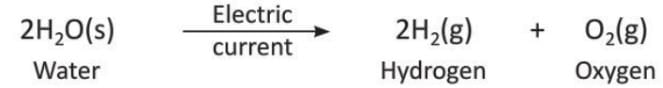
5. Electricity
- Some reactions happen when electricity is passed through the reactants.
- Example: Water breaks into hydrogen and oxygen when electricity is passed.
6. Pressure
- Some reactions happen under high pressure.
- Example: Nitrogen and hydrogen react under high pressure to form ammonia.
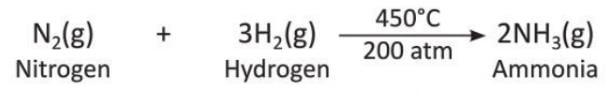
7. Catalyst
- Some reactions need a catalyst to happen. A catalyst is a substance that changes the speed of a reaction without changing itself.
Catalyst and Enzyme
Catalyst: A catalyst is a substance that changes the speed of a chemical reaction without changing itself.
Examples:
- Example 1: Manganese (IV) oxide helps hydrogen peroxide break down faster into water and oxygen.
Equation: 2H₂O₂(aq) → 2H₂O(l) + O₂(g)↑ (MnO₂ as catalyst)
Explanation: Manganese (IV) oxide speeds up the reaction where hydrogen peroxide breaks into water and oxygen. It shows effervescence (bubbles). - Example 2: Manganese (IV) oxide also speeds up the reaction of potassium chlorate to form oxygen.
Equation: 2KClO₃(s) → 2KCl(s) + 3O₂(g)↑ (MnO₂ as catalyst, 300°C)
Explanation: Potassium chlorate breaks into potassium chloride and oxygen faster with manganese (IV) oxide at 300°C. - Example 3: Photosynthesis in plants happens with chlorophyll as a catalyst.
Equation: 6CO₂(g) + 6H₂O(l) → C₆H₁₂O₆(aq) + 6O₂(g) (Chlorophyll as catalyst)
Explanation: Chlorophyll helps plants use sunlight to turn carbon dioxide and water into glucose and oxygen.
Types of catalysts
- Positive catalyst: Increases the speed of a reaction.
- Negative catalyst: Decreases the speed of a reaction.
Biocatalyst or Enzyme
- A substance that acts as a catalyst in living beings is called a biocatalyst or enzyme.
- Enzymes are made during biochemical reactions in living beings.
- Pepsin is an enzyme in the stomach that breaks proteins into amino acids.
- Trypsin and lipase are enzymes in the small intestine that break down starch, fats, and proteins.
- Enzyme maltase in yeast helps convert maltose (a carbohydrate) into glucose.
- Enzyme zymase in yeast helps ferment glucose into alcohol.
Did You Know?
Richard Martin Willstätter (1872-1942) was a German organic chemist. He won a Nobel Prize in 1915 for his research on plant pigments, especially chlorophyll.
Types of Chemical Reactions
Chemical reactions can be divided into many types based on how substances change.
The main types are:
- Combination reaction
- Decomposition reaction
- Displacement reaction
- Double displacement reaction
- Precipitation reaction
- Neutralisation reaction
- Redox reaction (Oxidation-reduction reaction)
Combination or Synthesis Reaction
- A reaction where two or more substances join to form a single new substance is called a combination reaction.
- It is also known as a synthesis reaction.
- The general form of this reaction is: A + B → AB
- Examples of combination reactions:
- Burning of magnesium: When magnesium burns in air, it joins with oxygen to form magnesium oxide, which is a white powder.

- Reaction of hydrogen and oxygen: Hydrogen combines with oxygen to form water.

- Formation of carbon and Oxygen: Carbon reacts with oxygen to form carbon dioxide.

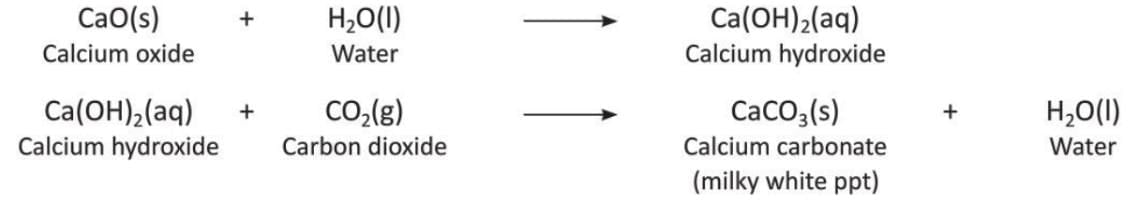
- Heating of calcium: When calcium is heated in the presence of oxygen, it burns with a brick-red flame to form calcium oxide.

- Reaction of calcium oxide and water: Calcium oxide combines with water to form calcium hydroxide.
- Burning of magnesium: When magnesium burns in air, it joins with oxygen to form magnesium oxide, which is a white powder.
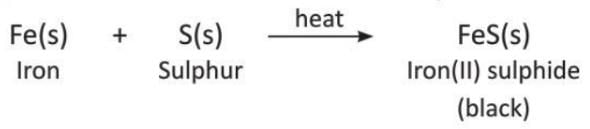
Activity 6.4
Aim: To show a combination reaction.
Materials required: A test tube, a burner, some powdered iron, some powdered sulphur.
Procedure:
- Take a small amount of finely powdered iron and sulphur in a test tube.
- Heat the test tube for some time.
Observation: You will see that a black compound is formed.
Conclusion: Iron and sulphur, when heated, combine to form iron(II) sulphide, which is black in colour.
Did You Know?
The digestion of food in our body is an example of a decomposition reaction. When we eat substances like wheat, rice, or potatoes, the starch present in them decomposes to form simple sugars like glucose and proteins decompose to form amino acids.
Decomposition Reaction
- A reaction where a compound breaks into two or more simpler substances is called a decomposition reaction.
- It is the opposite of a combination reaction.
- The general form of this reaction is: AB → A + B
- Decomposition caused by heat is called thermal decomposition.
- Examples of decomposition reactions:
- Thermal decomposition of limestone: When calcium carbonate (limestone) is heated, it breaks into calcium oxide (quick lime) and carbon dioxide gas.

- Thermal decomposition of potassium chlorate: Potassium chlorate, when heated with manganese dioxide, gives potassium chloride and oxygen gas.
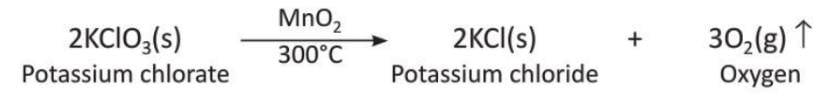
- Thermal decomposition of limestone: When calcium carbonate (limestone) is heated, it breaks into calcium oxide (quick lime) and carbon dioxide gas.
- More examples of thermal decomposition:

- Decomposition caused by electricity is called electrolytic decomposition.
- Examples:
- Decomposition of water: When an electric current passes through water, it breaks into hydrogen and oxygen gas.

- Decomposition of sodium chloride: When an electric current passes through a solution of sodium chloride, it breaks into sodium and chlorine gas.

- Decomposition of aluminium oxide: When an electric current passes through molten aluminium oxide, it breaks into aluminium and oxygen gas.
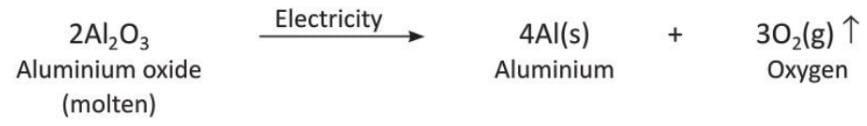
- Decomposition of water: When an electric current passes through water, it breaks into hydrogen and oxygen gas.
- Decomposition caused by light is called photolytic decomposition.
- Examples:
- Decomposition of silver chloride: When silver chloride is exposed to light, it breaks into silver metal and chlorine gas.

- Decomposition of silver bromide: When silver bromide is exposed to light, it breaks into silver and bromine gas.

- Decomposition of silver chloride: When silver chloride is exposed to light, it breaks into silver metal and chlorine gas.
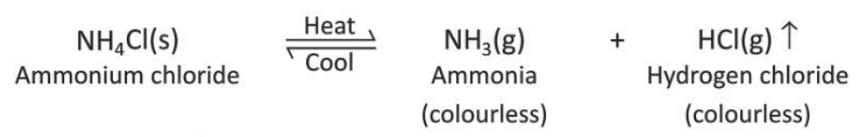
Activity 6.5
Aim: To show thermal decomposition of ammonium chloride.
Materials required: Some ammonium chloride, a test tube, a burner.
Procedure:
- Take a small amount of ammonium chloride in a test tube and heat it.
Observation: Ammonium chloride breaks into ammonia and hydrogen chloride gas on heating. White crystals of ammonium chloride are seen in the upper part of the test tube on cooling.
Conclusion: This shows that ammonium chloride on heating breaks to give ammonia and hydrogen chloride gases.
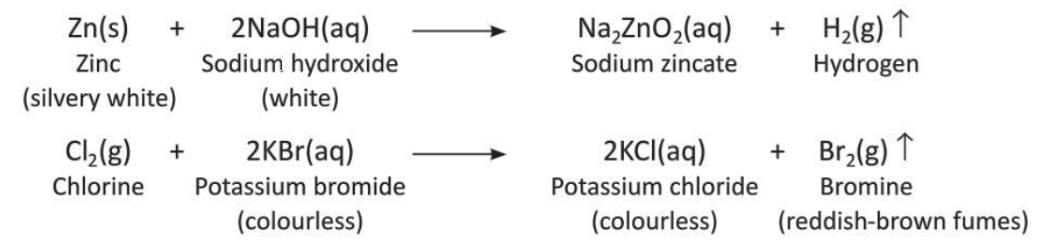
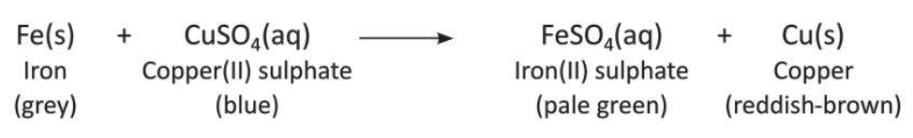
Displacement Reaction
- A reaction where one element takes the place of another element in a compound is called a displacement reaction.
- In this reaction, a more reactive metal replaces a less reactive metal from its compound, like in an acid, alkali, or water.
- The general form of this reaction is: AB + C → CB + A
- Examples of displacement reactions:
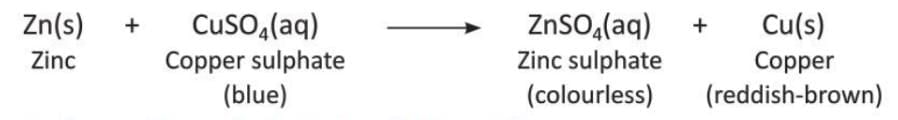
- Zinc displaces copper from a solution of copper(II) sulphate.
- Zinc displaces hydrogen from dilute hydrochloric acid because zinc is more reactive.

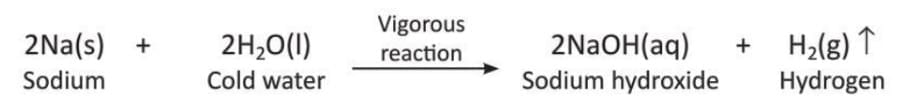
- Sodium displaces hydrogen from water: Active metals like sodium or potassium react with cold water to displace hydrogen. These reactions are very violent because of the reactive nature.
- Zinc displaces copper from a solution of copper(II) sulphate.
- More examples of displacement reactions:
Double Displacement Reaction
- A reaction where two compounds react by exchanging their ions to form two new compounds is called a double displacement reaction.
- The general form of this reaction is:

- Double displacement reactions are of two types: precipitation reaction and neutralisation reaction.
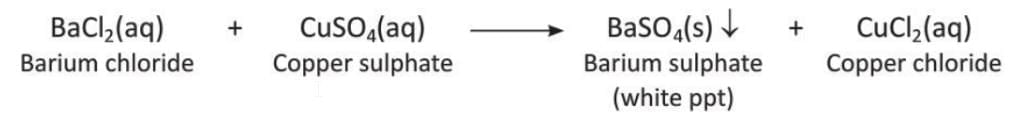
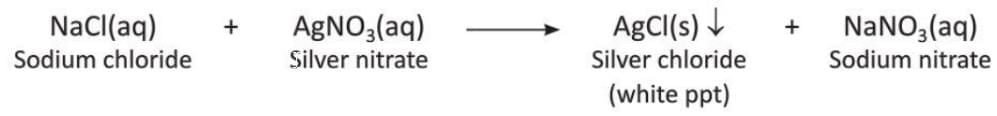
Precipitation Reaction
- A reaction where an insoluble solid called a precipitate separates from the solution is called a precipitation reaction.
- Examples of precipitation reactions:
- Reaction of barium chloride and copper sulphate: Barium chloride and copper sulphate in the form of a solution react to form a white precipitate of barium sulphate.
- Reaction of sodium chloride and silver nitrate: Sodium chloride and silver nitrate in the form of a solution react to form a white precipitate of silver chloride.
- Reaction of barium chloride and copper sulphate: Barium chloride and copper sulphate in the form of a solution react to form a white precipitate of barium sulphate.
- More examples of precipitation reactions:
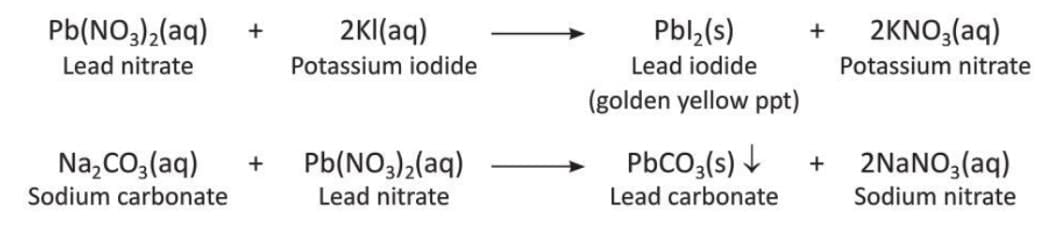
Neutralisation Reaction
- A reaction where an acid reacts with a base to form salt and water is called a neutralisation reaction.
- The general form of this reaction is: Acid + Base → Salt + Water
- Examples of neutralisation reactions:
- Reaction of sodium hydroxide and hydrochloric acid: Sodium hydroxide and dilute hydrochloric acid react to form sodium chloride salt and water.
HCl(aq) (Hydrochloric acid) + NaOH(aq) (Sodium hydroxide) → NaCl(aq) (Sodium chloride) + H₂O(l) (Water) - Reaction of potassium hydroxide and sulphuric acid: Potassium hydroxide reacts with dilute sulphuric acid to form potassium sulphate and water.
H₂SO₄(aq) (Sulphuric acid) + 2KOH(aq) (Potassium hydroxide) → K₂SO₄(aq) (Potassium sulphate) + 2H₂O(l) (Water) - Reaction of zinc oxide and nitric acid: Zinc oxide and nitric acid react to produce zinc nitrate and water.
ZnO(s) (Zinc oxide) + 2HNO₃(aq) (Dilute nitric acid) → Zn(NO₃)₂(aq) (Zinc nitrate) + H₂O(l) (Water)
- Reaction of sodium hydroxide and hydrochloric acid: Sodium hydroxide and dilute hydrochloric acid react to form sodium chloride salt and water.
- More examples of neutralisation reactions:
- CuO(s) (Copper(II) oxide) + dil. H₂SO₄(aq) (Dilute sulphuric acid) → CuSO₄(aq) (Copper sulphate) + H₂O(l) (Water)
- PbO(s) (Lead oxide) + 2HNO₃(aq) (Dilute nitric acid) → Pb(NO₃)₂(aq) (Lead nitrate) + H₂O(l) (Water)
- Zn(OH)₂(s) (Zinc hydroxide) + H₂SO₄(aq) (Sulphuric acid) → ZnSO₄(aq) (Zinc sulphate) + 2H₂O(l) (Water)
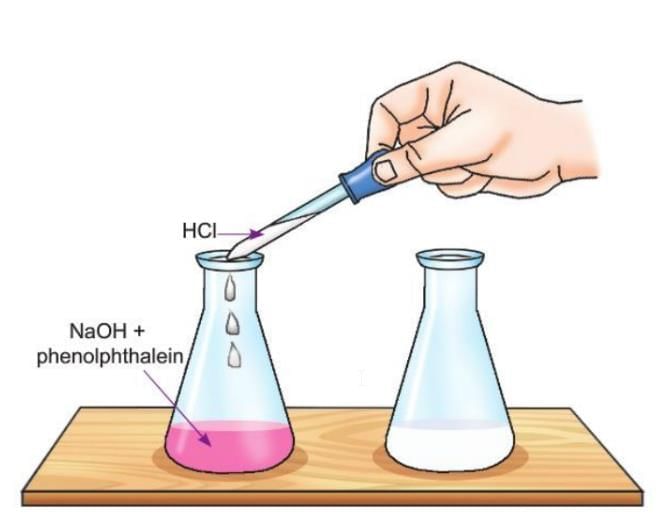
Activity 6.6
Aim: To show a neutralisation reaction.
Materials required: Some sodium hydroxide, a conical flask, a glass dropper, some phenolphthalein solution, dilute hydrochloric acid.
Procedure:
- Take about 50 ml of sodium hydroxide in a conical flask.
- Add 2-3 drops of phenolphthalein solution to it.
- Now add dilute hydrochloric acid dropwise with the help of a glass dropper. Shake well each time you add a drop.
Observation: The solution becomes colourless.
Now add a drop of dilute hydrochloric acid.
The solution again turns pink.
Conclusion: The stage when the base is completely neutralised by the acid is the neutralisation point, i.e., when the solution becomes colourless.
Importance of Neutralisation Reactions in Our Daily Life
- Acidity: When we eat too much spicy or oily food, our stomach makes extra acid called hydrochloric acid, causing a burning feeling.
- We take a medicine called antacid, like milk of magnesia (magnesium hydroxide), to feel better.
- The antacid reacts with the stomach acid to neutralise it and stop the burning.
- Ant and Bee Sting: When an ant or bee stings, it injects formic acid into our body, causing pain and itching.
- To feel better, we apply baking soda or calamine lotion on the sting area.
- Baking soda and calamine lotion are basic and neutralise the formic acid to reduce pain and itching.
- Soil Treatment: Farmers use fertilisers to help plants grow, but some fertilisers make the soil too acidic or too basic.
- If the soil is too acidic, farmers add quicklime (calcium oxide) or slaked lime (calcium hydroxide), which are basic, to neutralise the soil.
- If the soil is too basic, farmers add organic matter to make it neutral for better plant growth.
- Disposal of Factory Wastes: Factories release acidic wastes into water bodies, which can harm aquatic plants and animals.
- Basic substances are added to the acidic wastes to neutralise them before releasing into water bodies.
Redox Reaction
- A redox reaction is a chemical reaction where oxidation and reduction happen at the same time.
- Oxidation: It is when a substance gains oxygen or loses hydrogen.
- Example of Oxidation:
- 2Mg (Magnesium) + O₂ (Oxygen) → 2MgO (Magnesium oxide) when heated.
- Magnesium gains oxygen to form magnesium oxide, so this is oxidation.
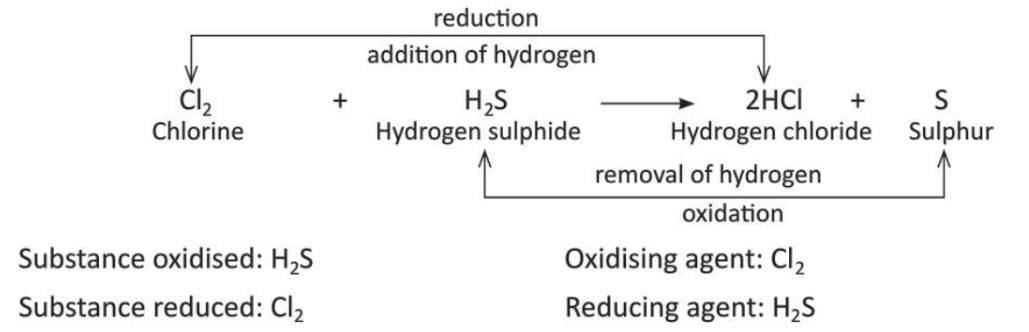
- H₂S (Hydrogen sulphide) + Cl₂ (Chlorine) → 2HCl (Hydrochloric acid) + S (Sulphur).
- Hydrogen sulphide loses hydrogen to form sulphur, so this is oxidation.
- Reduction: It is when a substance loses oxygen or gains hydrogen.
- Example of Reduction:
- H₂ (Hydrogen) + Cl₂ (Chlorine) → 2HCl (Hydrochloric acid).
- Chlorine gains hydrogen to form hydrochloric acid, so this is reduction.
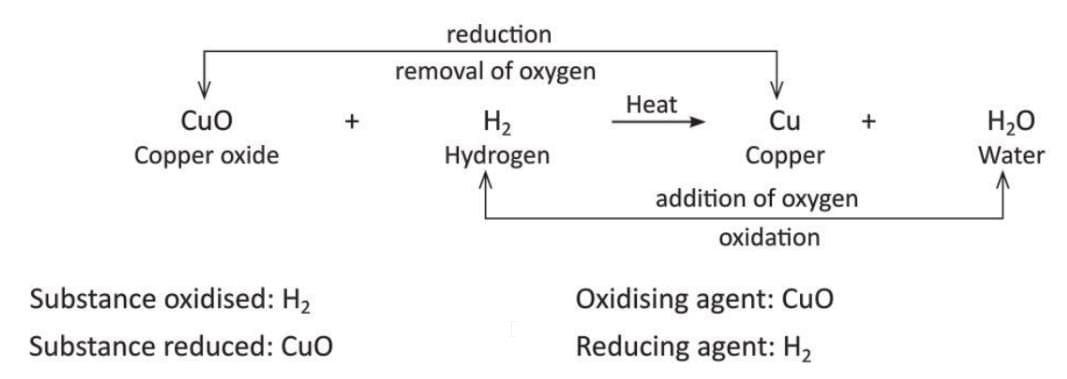
- CuO (Copper oxide) + H₂ (Hydrogen) → Cu (Copper) + H₂O (Water) when heated.
- Copper oxide loses oxygen to form copper, so this is reduction.
Oxidising and Reducing Agents
- Oxidising Agent: It is a substance that gives electrons to another substance during an oxidation reaction. Common oxidising agents are oxygen, ozone, chlorine, bromine, nitric acid, and sulphuric acid.
- Reducing Agent: It is a substance that loses electrons during a reduction reaction. Common reducing agents are hydrogen, carbon, and sulphur dioxide.
- Examples:
- Example 1: In this reaction, copper oxide loses oxygen (reduction), so copper oxide is the substance reduced. Hydrogen gains oxygen to form water (oxidation), so hydrogen is the substance oxidised. Copper oxide is the oxidising agent because it causes oxidation of hydrogen. Hydrogen is the reducing agent because it causes reduction of copper oxide.
- Example 2: In this reaction, hydrogen sulphide loses hydrogen to form sulphur (oxidation), so hydrogen sulphide is the substance oxidised. Chlorine gains hydrogen to form hydrochloric acid (reduction), so chlorine is the substance reduced. Chlorine is the oxidising agent because it causes oxidation of hydrogen sulphide. Hydrogen sulphide is the reducing agent because it causes reduction of chlorine.
- More Examples:
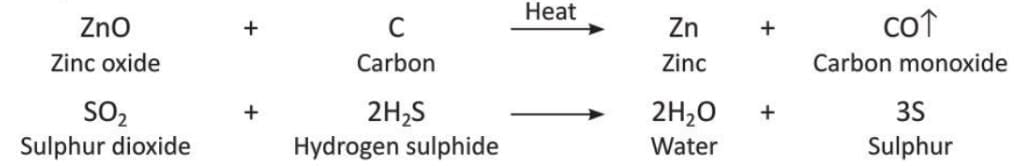
- Example 1: In this reaction, copper oxide loses oxygen (reduction), so copper oxide is the substance reduced. Hydrogen gains oxygen to form water (oxidation), so hydrogen is the substance oxidised. Copper oxide is the oxidising agent because it causes oxidation of hydrogen. Hydrogen is the reducing agent because it causes reduction of copper oxide.
Reactivity or Activity Series of Metals
- Some metals are very reactive, while others are less reactive or unreactive.
- Metals are arranged in a list called the reactivity series based on how reactive they are.
- In the reactivity series, the most reactive metal is at the top, and the least reactive metal is at the bottom.
- Reactivity Series of Metals:
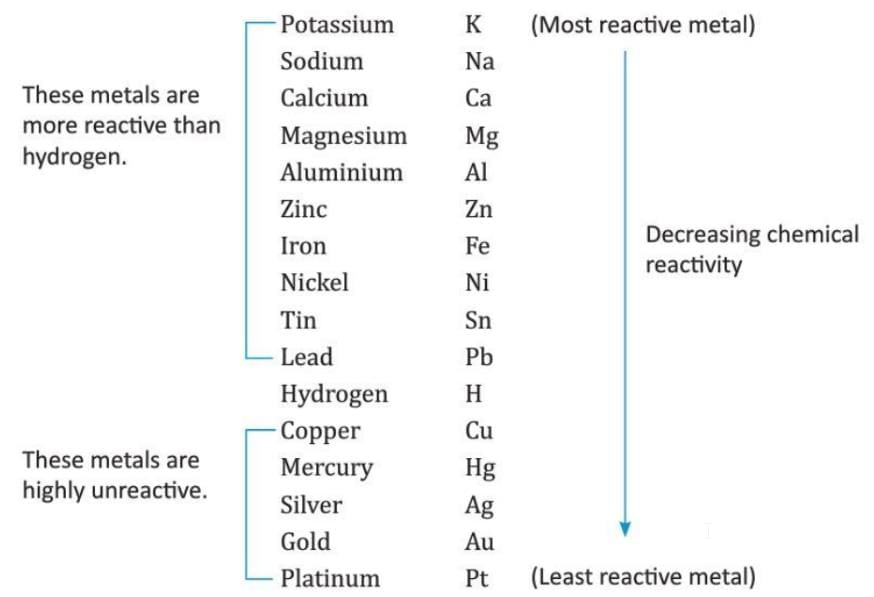
- Metals above hydrogen in the series are more reactive than hydrogen and can displace hydrogen from water, acids, or alkalis.
- Metals below hydrogen cannot displace hydrogen.
- Metals higher in the reactivity series can take part in reactions with oxygen or dilute acids more easily.
- Metals lower in the reactivity series do not react with oxygen or dilute acids easily.
Reactions of Metals with Oxygen, Water, and Acids
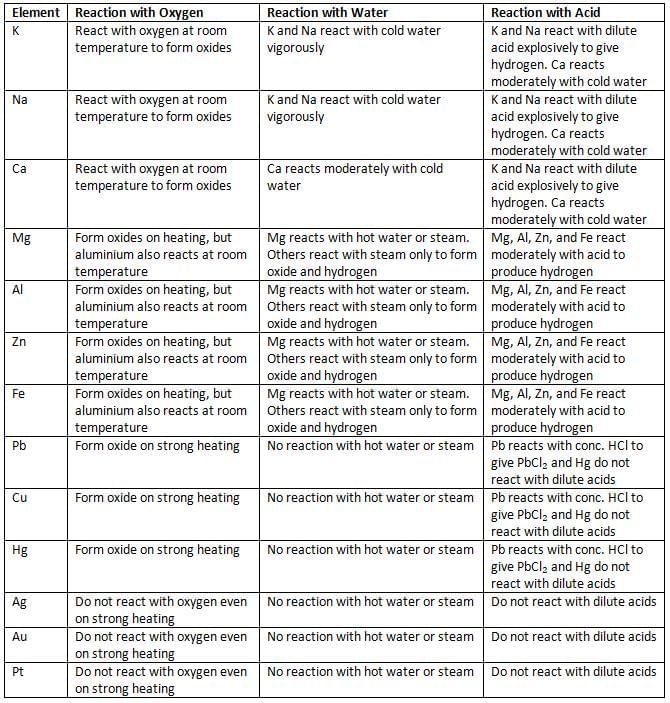
Decomposition Reactions to Form Oxides
- An oxide is a compound made of oxygen with a metal or non-metal.
- Metals combine with oxygen to form metal oxides, and non-metals form non-metal oxides.
- We can make oxides of metals by heating their ores.
- This process can be done in two ways: calcination and roasting.
Calcination
- Calcination is a process where a metal ore is heated strongly with a limited supply of air to remove carbon dioxide and water.
- This method is used for carbonate ores.
- Examples:
- CaCO₃ (Calcium carbonate) → CaO (Calcium oxide) + CO₂ (Carbon dioxide) when heated.
- ZnCO₃ (Zinc carbonate) → ZnO (Zinc oxide) + CO₂ (Carbon dioxide) when heated.
- CuCO₃·Cu(OH)₂ (Copper carbonate) → 2CuO (Copper oxide) + CO₂ (Carbon dioxide) + H₂O (Water) when heated.
Roasting
- Roasting is a process where a metal ore is heated strongly in the presence of air at a very high temperature.
- This method is used for sulphide ores.
- Sulphur dioxide is produced along with the metal oxide.
- Examples:
- 2PbS (Lead sulphide) + 3O₂ (Oxygen) → 2PbO (Lead oxide) + 2SO₂ (Sulphur dioxide).
- 4FeS (Iron sulphide) + 7O₂ (Oxygen) → 2Fe₂O₃ (Iron oxide) + 4SO₂ (Sulphur dioxide).
- 2Cu₂S (Copper sulphide) + 3O₂ (Oxygen) → 2Cu₂O (Copper oxide) + 2SO₂ (Sulphur dioxide).
Decomposition of Nitrates
- Metal nitrates break down on heating to give metal oxides, nitrogen dioxide, and oxygen gas.
- Examples:
- 2Pb(NO₃)₂ (Lead nitrate) → 2PbO (Lead oxide) + 4NO₂ (Nitrogen dioxide) + O₂ (Oxygen) when heated.
- 2Mg(NO₃)₂ (Magnesium nitrate) → 2MgO (Magnesium oxide) + 4NO₂ (Nitrogen dioxide) + O₂ (Oxygen) when heated.
Classification of Oxides
Oxides are of four types based on their nature and properties:
1. Acidic Oxides
- Acidic oxides or non-metal oxides are formed when non-metals are heated in the presence of oxygen.
- Non-metals like carbon, sulphur, and nitrogen combine with oxygen to form their oxides.
- Examples:
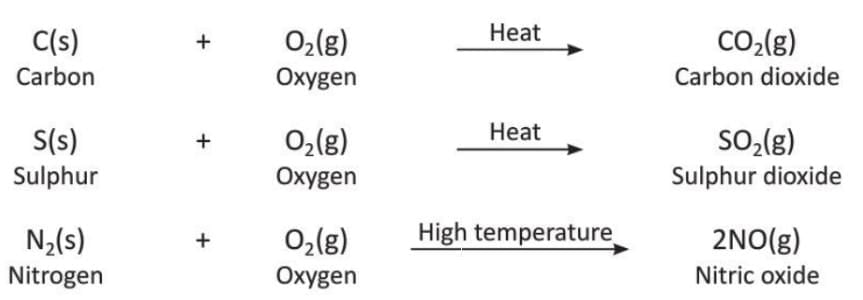
- Most acidic oxides turn moist blue litmus paper red.
- Acidic oxides are usually gases at room temperature.
- Acidic oxides dissolve in water to form acids.
- Examples:
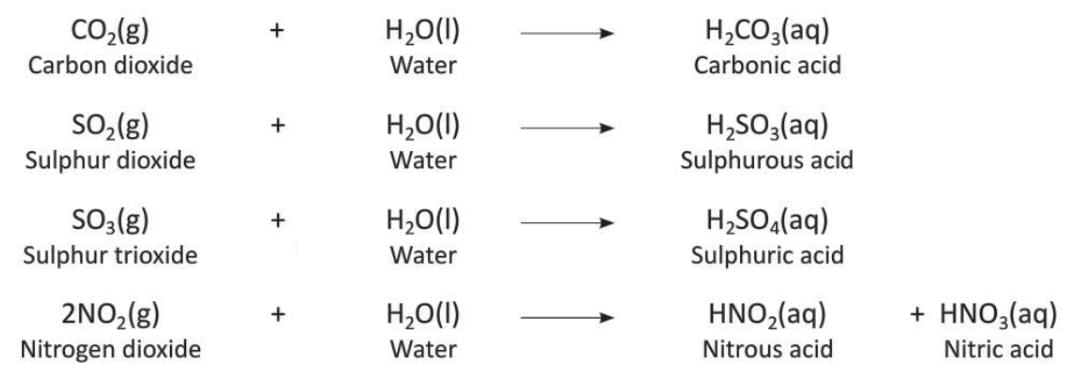
- Acidic oxides react with bases and alkalis to form salt and water.
- Examples:
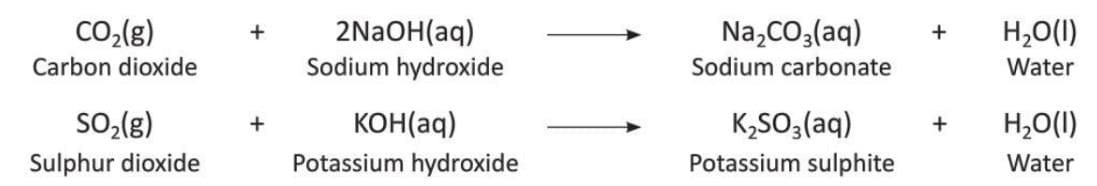
2. Basic Oxides
- Basic oxides or metal oxides are formed when metals react with oxygen on heating or without heating.
- Some basic oxides are listed below with their formulas:
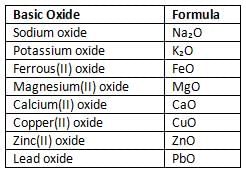
- Most basic oxides exist as solids at room temperature.
- Basic oxides are generally insoluble in water but dissolve in water to form soluble bases called alkalis.
- Examples:
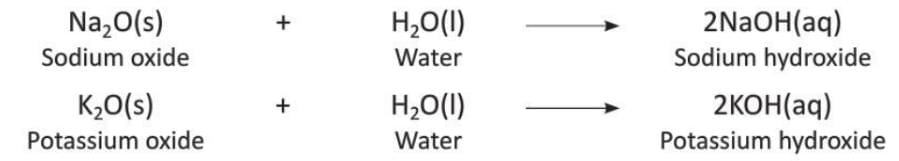
- Both sodium hydroxide and potassium hydroxide are strong alkalis and turn litmus paper blue.
Note: All alkalis are bases, but all bases are not alkalis.
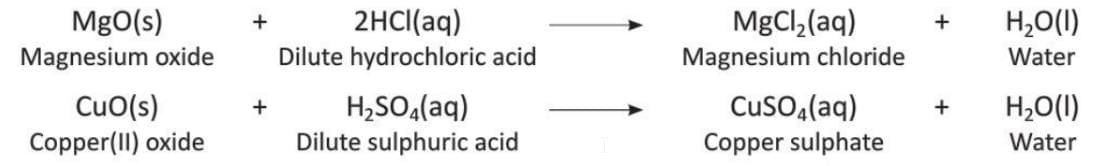
3. Salts
- Basic oxides react with acids to form salts.
- Examples:
4. Neutral Oxides
- Oxides that do not show either acidic or basic properties are called neutral oxides.
- Carbon monoxide (CO) and nitrous oxide (NO) are neutral and do not change the colour of indicators.
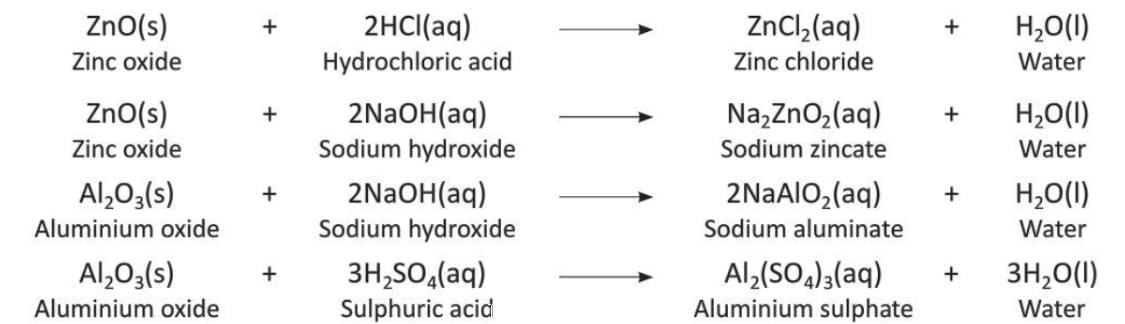
5. Amphoteric Oxides
- Oxides like zinc oxide and aluminium oxide can react with both acids and bases to form salt and water.
- These are called amphoteric oxides.
- Examples:
Points To Remember
- A chemical change happens when atoms rearrange to form new substances with different properties.
- During the formation of new substances, there may be a chemical change, gas evolution, colour change, formation of a precipitate, or change of state or energy.
- Chemical reactions can be exothermic (release heat) or endothermic (absorb heat).
- Chemical reactions need conditions like heat, light, electricity, pressure, and catalysts to happen.
- Catalysts are of two types: positive catalysts (increase reaction rate) and negative catalysts (decrease reaction rate).
- Life processes in our body like digestion are due to biochemical reactions.
- Enzymes like amylase, trypsin, lipase, and pepsin help in these reactions.
- Chemical reactions can be combination, decomposition, displacement, double displacement, or redox (oxidation-reduction) reactions.
- Examples of combination reactions: burning of magnesium, reaction of calcium oxide with water, and reaction of carbon with oxygen.
- Examples of decomposition reactions: decomposition of limestone, potassium chlorate, copper hydroxide, and lead nitrate when heated.
- Examples of thermal decomposition: decomposition of water and aluminium oxide when heated.
- Examples of electric decomposition: decomposition of silver chloride and silver bromide when exposed to light.
- Examples of displacement reactions: a more reactive metal displaces a less reactive metal from its compound, like zinc displacing copper from copper sulphate to form zinc sulphate.
- Examples of double displacement reactions: reaction of barium chloride with sodium sulphate, reaction of lead nitrate with potassium iodide, and reaction of sodium carbonate with calcium chloride.
- Examples of neutralisation reactions: reaction of sodium hydroxide with hydrochloric acid, reaction of potassium hydroxide with sulphuric acid, reaction of zinc oxide with hydrochloric acid, and reaction of copper oxide with sulphuric acid.
- Redox reactions involve both oxidation and reduction happening together.
- Based on the vigour of reactions with oxygen, water, and acids, metals are arranged in a reactivity series.
- In the reactivity series, the most reactive metal is at the top, and the least reactive metal is at the bottom.
- Oxygen combines with metals and non-metals to form oxides.
- Oxides of metals can be produced by heating their ores in two ways: calcination and roasting.
- Oxides are of four types: acidic oxides, basic oxides, amphoteric oxides, and neutral oxides.
Glossary
- Chemical reaction: A process in which new substance(s) with new properties is/are formed
- Reactants: Substances that take part[z in a chemical reaction
- Products: Substances that are produced in a chemical reaction
- Exothermic reaction: The chemical reaction in which heat energy is evolved
- Endothermic reaction: The chemical reaction in which heat energy is absorbed
- Photochemical reaction: A chemical reaction that takes place in the presence of light
- Electrochemical reaction: A chemical reaction that occurs when the electricity is passed through the reactants
- Catalyst: A substance that alters the rate of a chemical reaction without itself undergoing any chemical change during the reaction
- Positive catalyst: A catalyst that increases the rate of a chemical reaction
- Negative catalyst: A catalyst that decreases the rate of a chemical reaction
- Biocatalyst or enzyme: A substance that acts as a catalyst in a biochemical reaction
- Combination or synthesis reaction: A chemical reaction in which two or more substances combine to form a single substance
- Decomposition reaction: A chemical reaction in which a compound splits into two or more simpler substances
- Thermal decomposition: A decomposition reaction brought about by heat
- Electric decomposition: A decomposition reaction brought about by electricity
- Photodecomposition: A decomposition reaction brought about by light
- Displacement reaction: A chemical reaction in which an element takes the place of another element in a compound
- Double displacement reaction: A chemical reaction in which two compounds react by exchange of ions to form two new compounds
- Precipitation reaction: A chemical reaction in which an insoluble solid called precipitate separates from the solution
- Neutralisation reaction: A chemical reaction in which an acid reacts with a base to form salt and water
- Redox reaction: A chemical reaction in which oxidation and reduction take place simultaneously
- Oxidation: The addition of oxygen or the removal of hydrogen from a substance
- Reduction: The addition of hydrogen or the removal of oxygen from a substance
- Oxidising agent: An atom or a group of atoms which accepts electrons during an oxidation reaction
- Reducing agent: An atom or a group of atoms which loses electrons during a chemical reaction
- Reactivity series of metals: The arrangement of metals in a vertical column in the order of their decreasing reactivity
- Oxide: A compound which consists of oxygen as an anion
- Calcination: A decomposition process in which a metallic ore is heated in the absence or limited supply of air
- Roasting: A decomposition process in which a metallic ore is heated in the presence of air at a very high temperature
- Oxides formed when non-metals react with oxygen on heating: Oxides formed when non-metals react with oxygen on heating or short Amphoteric oxides
- Amphoteric oxides: Oxides that can react with both acids and bases to form salt and water
- Neutral oxides: Oxides which do not exhibit either acidic or basic properties
|
9 videos|46 docs|9 tests
|
FAQs on Chemical Reactions Chapter Notes - Chemistry Class 8 ICSE
| 1. What are the different types of chemical reactions? |  |
| 2. Why are neutralization reactions important in our daily life? |  |
| 3. What is the reactivity series of metals? |  |
| 4. How do decomposition reactions form oxides? |  |
| 5. What are the classifications of oxides? |  |