Corynebacterium and Bacillus Chapter Notes | Microbiology - NEET PG PDF Download
Corynebacterium
Corynebacterium is a type of bacteria that is shaped like a club, is gram-positive, and does not have a capsule, spores, or the ability to move on its own.
- Corynebacterium diphtheriae, also known as the Klebs-Loeffler bacillus, has some unique features. It is known for its distinctive way of appearing in smears, where it looks like Chinese letters or cuneiform shapes (V or L shaped). This is because the bacteria divide in a way called snapping division, where the daughter cells remain at sharp angles to each other.
- Metachromatic granules, also known as polar bodies, Babes Ernst bodies, or volutin granules, are storage granules found at the ends or poles of bacilli.
- These granules are made of polymetaphosphates and stain better with special dyes such as Albert’s, Neisser’s, and Ponder’s stain.
- They are well developed on enriched media like blood agar or Loeffler’s serum slope.
- Volutin granules can also be possessed by:
- Corynebacterium xerosis
- Gardnerella vaginalis
- Spirillium
- Few Mycobacterium species
- Enterobacter aerogene
- Few yeasts.
Virulence Factor: Diphtheria Toxin
Mechanism of Action
- DT is the main factor that causes diphtheria:
- It consists of two parts: Fragment A (which is the active part) and Fragment B (which is the binding part).
- Fragment B attaches to the receptors on host cells (like epidermal growth factor) to help Fragment A enter the cells.
- Once inside the host cells,Fragment A:
- causes ADP ribosylation of elongation factor 2 (EF2),
- leads to the inhibition of EF2,
- results in stopping the translation process of protein production,
- ultimately causes cell death.
- The way DT works is similar to the exotoxin A from Pseudomonas.
Toxin Production relies on:
- Phage coded: DT is produced by a β corynephage that carries the tox gene.
- Iron concentration: The production of the toxin depends on the right level of iron (0.1 mg per liter). If there is too much iron, it stops toxin production by activating the DT repressor gene in the bacteria's chromosome.
- The DT repressor gene (DtxR) is a negative regulator that controls both DT production and iron absorption in C. diphtheriae.
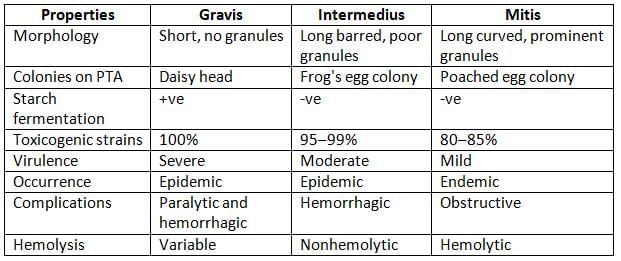
- Biotypes: Among the three biotypes of C. diphtheriae, all strains of gravis, 95-99% of intermedius strains, and 80-85% of mitis strains are capable of producing toxins. However, the toxins from different biotypes are similar in their antigenic properties.
- Other species: DT is also produced by C. ulcerans and C. pseudotuberculosis.
Toxoid is used for Vaccination
- Diphtheria toxin is recognized by the immune system, and antitoxins can offer protection. However, because it is harmful, it cannot be used directly for vaccines.
- The toxin can be turned into a toxoid, which is what is used for vaccination. A toxoid is a modified version of the toxin that loses its harmful effects but still maintains its ability to trigger an immune response.
- The process of creating a toxoid is enhanced by using formalin, maintaining an acidic pH, and storing it for a long time.
- The Park William 8 strain of C. diphtheriae is the source of the toxin used to make the vaccine.
- The strength of the toxin is measured in Loeffler’s flocculating unit (Lf unit). One Lf unit is defined as the amount of toxin that causes the fastest clumping when mixed with one unit of antitoxin.
Pathogenicity and Clinical Manifestations
Diphtheria is a type of illness caused by toxins but not by bacteria spreading in the blood.
- Bacilli (the bacteria) do not invade the body; they only stay in the throat area (pharynx) and release toxins.
- These toxins travel through the bloodstream, affecting various organs.
- The toxin is responsible for all symptoms, including local issues in the respiratory system and more serious systemic problems (except for skin problems which can be caused by the bacteria itself).
Respiratory Diphtheria
- This is the most frequent type of diphtheria.
- The common areas affected are the tonsils and the pharynx (faucial diphtheria), followed by the nose and larynx.
- In rare cases, it can affect non-respiratory areas like the conjunctiva (eyes) or vagina.
- The incubation period is usually around 3 to 4 days.
- Faucial diphtheria: The toxin causes inflammation, leading to tissue death and the formation of an exudate that creates a pseudomembrane.
- The pseudomembrane is a tough, leathery, greyish-white layer made of a core of fibrin surrounded by neutrophils, red blood cells, and bacteria.
- This pseudomembrane sticks to the mucosal surface and can bleed when removed, unlike a true membrane (like in Candida) which comes off easily.
- Extension of the pseudomembrane: In severe cases, it may spread to the larynx and medium-sized bronchial passages, potentially causing life-threatening airway blockage (asphyxia), which requires immediate tracheostomy.
- Bull-neck appearance: This is marked by significant swelling of the tonsils and neck edema.
Cutaneous Diphtheria
- This type shows punched-out ulcerative lesions with tissue death, and sometimes a pseudomembrane.
- Cutaneous diphtheria is caused directly by the bacteria and not by the toxins.
- Skin issues can also arise from non-toxic strains of the bacteria.
- There is a growing occurrence of cutaneous diphtheria, particularly among vaccinated children, because the antitoxins in vaccinated individuals do not prevent the disease.
Systemic Complications
- Neurological problems can occur, including issues with cranial nerves, peripheral neuropathy, and ciliary paralysis.
- Myocarditis is another late toxic effect that can develop weeks after the initial infection.
Pseudomembrane in Diphtheria
- The pseudomembrane in diphtheria appears as a tough, leathery coat that is greyish-white in color.
- It is composed of an inner band of fibrin, which is surrounded by neutrophils, red blood cells (RBCs), and bacteria.
- This membrane is adherent to the tissue and bleeds upon removal.
Laboratory Diagnosis of Diphtheria
- The diagnosis of diphtheria relies on observing clinical signs and symptoms, along with confirmation from laboratory tests.
- Due to the potential risk of breathing difficulties, specific treatment should begin right away if diphtheria is suspected, without waiting for lab results.
- Laboratory tests are important only for:
- Confirming the clinical diagnosis
- Starting necessary control measures
- Gathering information for epidemiological studies
- The laboratory diagnosis involves:
- Isolating the bacilli
- Demonstrating the presence of the toxin.
Isolation of the Diphtheria Bacillus
- Specimen: Throat swabs (one or two) containing fibrinous exudates and a portion of the membrane
- Direct smear microscopy:
- Gram stain: C. diphtheriae appears as irregularly stained, club-shaped, gram-positive bacilli arranged in a Chinese letter or cuneiform formation (V or L shaped). Differentiating from other coryneforms in the respiratory tract is challenging.
- Albert’s stain: Traditionally used for C. diphtheriae; however, it's not the only method. Recent molecular techniques may offer more accurate identification, showing green bacilli with bluish-black metachromatic granules.
- Culture media:
- Enriched medium: such as Loeffler’s serum slope:
- Advantages:
- Detects growth early (6–8 hrs)
- Best medium for metachromatic granules production
- Disadvantage:
- Incubation beyond 6–8 hrs may also support the growth of other throat commensals.
- Selective medium: such as Potassium tellurite agar (PTA) and Tinsdale medium:
- Advantage: Inhibits throat commensals; hence, these are the best media for isolating C. diphtheriae from both cases and carriers.
- Disadvantage: Black colonies appear only after 48 hours of incubation.
- Biochemical identification:
- Hiss’s serum sugar media: C. diphtheriae ferments glucose and maltose (by all biotypes) and starch (by only gravis).
- Pyrazinamidase test: Negative for C. diphtheriae, C. ulcerans, and C. pseudotuberculosis.
- Urease test: Negative for C. diphtheriae; C. ulcerans and C. pseudotuberculosis are urease positive.
- Corynebacterium: Catalase positive but oxidase negative and nonmotile.
Demonstrating the Diphtheria Toxin
Diphtheria is caused by the diphtheria toxin, so merely isolating the bacilli is not enough for a complete diagnosis. After isolation, it is crucial to demonstrate the presence of the toxin, which can be done either in vivo (within a living organism) or in vitro (in a controlled environment outside a living organism).
In vivo tests using guinea pig inoculation can include:
- Subcutaneous test (injection under the skin)
- Intracutaneous inoculation (injection into the skin)
In vitro tests:
- Elek's gel precipitation test
- Detection of toxin gene by PCR (Polymerase Chain Reaction)
- Detection of diphtheria toxin by ELISA (Enzyme-Linked Immunosorbent Assay) or immunochromatographic test (ICT)
- Assessment of cytotoxicity in cell lines
Typing of C. diphtheriae
- Biotyping (McLeod’s classification): C. diphtheriae can be classified into four biotypes:
- Other methods: Serotyping, Bacteriophage typing, Bacteriocin typing, Molecular typing methods, such as PFGE.
C. diphtheriae biotypes
Epidemiology
- Diphtheria is an ancient disease that still occurs in certain regions, but thanks to widespread vaccination, cases have significantly decreased in most developed countries and some developing nations like India.
- The majority of infections (95%) are caused by carriers rather than sick individuals (5%).
- Carriers can be either temporary, lasting up to a month, or chronic, lasting up to a year.
- Nasal carriers pose a greater risk than throat carriers because they release the bacteria more frequently.
- The carrier rate varies between 0.1% and 5%.
- Diphtheria primarily spreads through the aerosol route and, less commonly, through contact with infected skin.
- Humans are the sole reservoir for this disease.
- Diphtheria is most prevalent in children aged 1–5 years.
- As immunization has increased, cases are shifting from preschool children to school-aged children.
- Newborns typically receive protection from their mothers' antibodies.
Treatment
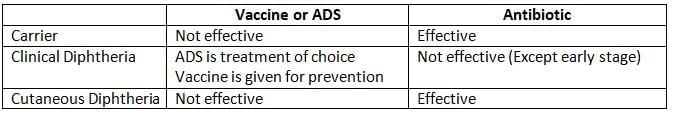
- Treatment should begin right away if diphtheria is suspected:
- Antidiphtheritic horse serum or ADS (antitoxin): This is the best treatment because it neutralizes the toxin.
- Antibiotics: Penicillin or erythromycin are the preferred medications. While antibiotics are not very helpful once the toxin is released, they can be beneficial in certain situations:
- If given early (within 6 hours of infection), they can work before the toxin is released.
- They can stop more toxin from being released by killing the bacteria.
- They are also important for treating cutaneous diphtheria.
- Treatment of carriers: Drug of choice is erythromycin
Prophylaxis (Vaccination)
- Active immunization uses diphtheria toxoid to stimulate the body to produce antitoxins.
- A protective level of antitoxin is considered to be above 0.01 Units/ml.
- To effectively stop the spread of diphtheria, herd immunity levels of around 80-90% are necessary.
- However, the vaccine is not effective for:
- Preventing cutaneous diphtheria.
- Eliminating the carrier stage.
- The available types of vaccines include:
- Single vaccine: Diphtheria toxoid (either alum-precipitated or formaldehyde-precipitated)
- Combined vaccine:
- DPT: Contains DT (diphtheria toxoid), Pertussis (whole cell), and TT (tetanus toxoid)
- DaPT: Contains DT, TT, and acellular pertussis (aP)
- DT: Contains DT and TT
- dT: Contains adult dose diphtheria toxoid (d) and TT
DPT Vaccine
- DPT is the preferred vaccine for infants because:
- It allows infants to be immunized against three major childhood diseases: diphtheria, tetanus, and pertussis with a single injection.
- The pertussis component enhances the effectiveness of the diphtheria and tetanus components.
- There are two types of DPT:
- Plain formol toxoid (or fluid toxoid): This is made by treating the toxin with formalin.
- Adsorbed (alum adsorbed): Here, alum is used as an adjuvant to boost the effectiveness of the toxoid.
- Administration of DPT:
- Schedule: According to India's national immunization schedule, a total of five doses are given: three doses at 6, 10, and 14 weeks of age, followed by two booster doses at 16-24 months and 5 years.
- Site: DPT is given as a deep intramuscular injection in the anterolateral area of the thigh. The gluteal area is not preferred because fat can affect DPT absorption.
- Thiomersal (0.01%) is used as a preservative.
- Storage: DPT should be stored at a temperature of 2-8°C. If it is accidentally frozen, it must be discarded.
- Dose: Each dose (0.5 ml) contains:
- Glaxo: 25 Lf (DT), 5 Lf (TT), 20,000 million (killed pertussis bacteria)
- Kasauli: 30 Lf (DT), 10 Lf (TT), 32,000 million (killed pertussis bacteria)
- dT: This contains tetanus toxoid and an adult dose (2 Lf) of diphtheria toxoid. It is given to adults over 12 years old in three doses at 0, 1 month, and 1 year.
- Reactions after DPT administration:
- Mild reactions: Commonly include fever and local reactions such as swelling and hardening.
- Severe reactions: The whole cell killed Bordetella pertussis can lead to encephalitis, which may cause neurological issues. For this reason, DPT is not recommended for children older than 6 years.
- Absolute contraindications to DPT:
- Hypersensitivity to a previous dose.
- Progressive neurological disorder.
The acellular pertussis component (aP) does not cause neurological complications and can be safely given to older children.
Schick test: This was a toxin-antitoxin neutralization test that is no longer used:
- It was used in the past when the vaccine was first introduced.
- The test was performed to identify individuals who were susceptible before they received immunization.
- Those who were susceptible developed redness and swelling at the test site after the intradermal injection of the toxin. The vaccine was only given to these susceptible individuals.
- Since safer toxoid preparations are now available, the Schick test is no longer performed.
Nondiphtheria Corynebacterium
- Clinical diphtheria:Corynebacterium ulcerans and Corynebacterium pseudotuberculosis can produce diphtheria toxin (DT) and cause clinical diphtheria. These species are urease positive and primarily infect animals.
- Corynebacterium ulcerans: This species mimics respiratory diphtheria and is transmitted through contaminated cow milk.
- Corynebacterium pseudotuberculosis, also known as Preisz Nocard bacilli, primarily affects sheep and horses.
- Corynebacterium minutissimum: This species causes a skin condition called erythrasma, which appears coral red under a Wood's lamp examination.
- Corynebacterium jeikeium: This is a multidrug-resistant species that can cause opportunistic infections in humans, particularly in those with weakened immune systems.
- Corynebacterium parvum: This species is an example of an immunomodulator and is known for its ability to modulate immune responses.
Bacillus
Bacilli that form spores are categorized into two groups:
- Bacillus: This group includes obligate aerobes that produce non-bulging spores.
- Clostridium: These are obligate anaerobes known for their bulging spores.
Bacillus Anthracis
B. anthracis is responsible for causing anthrax, a severe zoonotic disease. Its significance has increased recently due to its potential as a biological weapon.
Virulence Factors and Pathogenesis
The cause of anthrax is linked to two main factors that help it survive: the anthrax toxin and the capsule.
Anthrax Toxin
- Tripartite Toxin: The toxin has three parts:
- Edema Factor: This part is active and works as adenylcyclase, which increases the level of cyclic AMP in host cells.
- Protective Factor: This part binds to the receptor on host cells.
- Lethal Factor: This part causes cell death and stops the mitogen-activated protein kinase pathway.
- Combined Effect: When these three parts work together, they cause swelling in the area and can lead to widespread shock.
- Control of Toxin Synthesis: The production of the toxin is managed by a plasmid known as pX01. If this plasmid is lost, the strain becomes non-virulent. This principle was likely used to create the first anthrax vaccine by Pasteur.
- Live Attenuated Spore Vaccine: The Sterne and Mazucchi vaccines are made by removing the genes responsible for the capsule.
Anthrax Capsule
The bacterium B. anthracis has a capsule made of polypeptides, which are different from the polysaccharide capsules found in other bacteria.
- Capsule Coding: The capsule is also controlled by a plasmid called pX02.
- Function: This capsule helps prevent phagocytosis by complement proteins.
Pathogenesis and Clinical Manifestations
Anthrax mainly affects animals that eat plants. Animals like cattle, sheep, and sometimes horses and pigs are more likely to get infected compared to meat-eating animals.
Human Transmission
Humans can get infected in several ways:
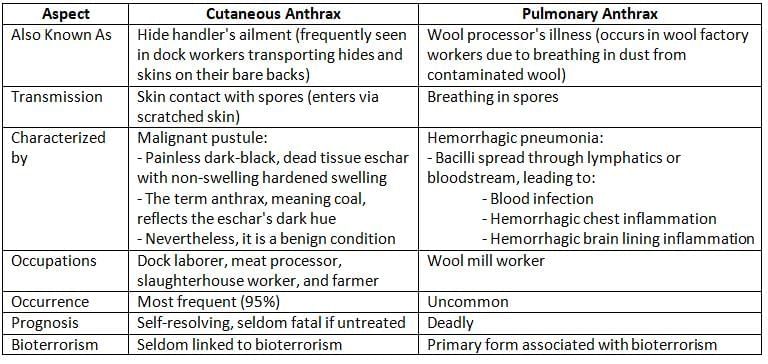
- Cutaneous Mode: Spores can enter through cuts in the skin, which is common among people who work with animals.
- Inhalation: Breathing in spores can lead to infection.
- Ingestion: Eating the meat of animals that died from anthrax, which may cause bloody diarrhea, can also transmit the disease.
Types of Human Anthrax
- Cutaneous Anthrax
- Pulmonary Anthrax
- Intestinal Anthrax
B. anthracis and Bioterrorism
- B. anthracis has been a major concern in bioterrorism and biological warfare, with significant outbreaks recorded in Sverdlovsk in 1979 and in the United States in 2001.
- During these outbreaks, pulmonary anthrax was the most frequently observed type.
- Pulmonary anthrax occurs when anthrax spores are inhaled, typically from contaminated animal products.
Laboratory Diagnosis: Distinguishing Anthrax from Anthracoid Bacilli
B. anthracis can be differentiated from other Bacillus species, known as Anthracoid Bacilli, through several key characteristics:
- Nonmotility and Capsule: B. anthracis is nonmotile and possesses a capsule.
- McFadyean Reaction: This reaction involves staining with polychrome methylene blue, revealing an amorphous purple capsule around blue bacilli.
- Gram Staining: Gram staining shows bacilli arranged in a pattern resembling bamboo sticks.
- Agar Plate Observation: Colonies exhibit a Medusa head appearance under low power magnification.
- Gelatin Stab Test: This test shows an inverted fir tree appearance.
- Solid Media with Penicillin: Cultures display a string of pearls appearance in the smear.
- Blood Agar: Colonies are non-hemolytic.
- Selective Medium: PLET media is used for selective cultivation.
- Direct Fluorescent Antibody Test (DFA): This test detects capsular antigens, confirming diagnosis during bioterrorism incidents.
- Ascoli’s Thermo Precipitin Test: This ring precipitation test identifies anthrax antigens.
- Spore Visualization: Spores can be observed using phase contrast microscopy or special stains like hot malachite green (Ashby’s method) or 0.25% sulfuric acid, as spores are acid-fast.
- Lipid Granule Demonstration: Lipid granules can be demonstrated using Sudan black B (Burdon’s method).
CDC Guidelines for Diagnosing Anthrax in Bioterrorism Scenarios (2001)
Following 2001 USA attack, CDC has prepared guidelines for identification of B. anthracis during bioterrorism.
- For presumptive identification of anthrax: Any large gram-positive bacillus with morphology and cultural properties similar to anthrax bacillus
- For initial confirmation, the tests done are Lysis by gamma phage and DFA
- Further confirmation is done by PCR.
Treatment
Anthrax can be successfully treated if the disease is promptly recognized and appropriate therapy is initiated early.
- Antibiotics for treatment: Consists of ciprofloxacin or doxycycline, plus clindamycin, and/or rifampin, for 60 days.
- Antibiotics for post-exposure prophylaxis:
- Ciprofloxacin for 60 days and
- Doxycycline, for 60 days or Amoxicillin, for 60 days
- Raxibacumab: It is a monoclonal antibody that neutralizes anthrax toxin (protective antigen). It is intended for the prophylaxis and treatment of inhaled anthrax.
Prevention
- Live attenuated, noncapsulated spore vaccine (Stern vaccine): It is extensively used in animals, but not for human use.
- Alum precipitated toxoid vaccine: It is prepared from the protective antigen. It is safe and effective for human use.
Other Bacillus Species
Bacillus Cereus
It is a normal habitant of soil, also widely isolated from food items, such as vegetables, milk, cereals, spices, meat and poultry.
Manifestations:
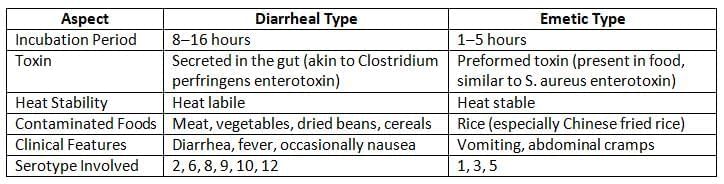
- Food poisoning: It produces two kinds of toxins:
- Diarrheal toxin
- Emetic toxin
- Ocular disease: Can cause severe keratitis and panophthalmitis after eye injuries.
- Other conditions: Though rare, it can lead to systemic infections such as:
- Endocarditis
- Meningitis
- Osteomyelitis
- Pneumonia
Laboratory Diagnosis: B. cereus is capable of moving, does not have a capsule, and is not affected by gamma phage. It can be found in feces using special media, such as:
- MYPA: Contains mannitol, egg yolk, phenol red, polymyxin, and agar.
- PEMBA: Contains polymyxin B, egg yolk, mannitol, bromothymol blue, and agar.
Treatment
B. cereus is treatable with:
- Clindamycin
- Erythromycin
- Vancomycin
However, it is resistant to penicillin (due to the production of β-lactamase) and trimethoprim.
Bacillus Thuringiensis
It can sometimes cause food poisoning. It is also used to control mosquito larvae.
Bacillus for Sterilization Control
- B. stearothermophilus and B. subtilis: Both are used as biological indicators for autoclave and plasma sterilization.
- B. pumilus: This bacterium serves as a biological control for ionizing radiation.
- B. globigii: This type is used to monitor ethylene oxide sterilization processes.
|
75 docs|5 tests
|
FAQs on Corynebacterium and Bacillus Chapter Notes - Microbiology - NEET PG
| 1. What is the mechanism of action of diphtheria toxin produced by Corynebacterium diphtheriae? |  |
| 2. What are the common clinical manifestations of diphtheria? |  |
| 3. How is Corynebacterium diphtheriae diagnosed in the laboratory? |  |
| 4. What are the primary preventive measures against diphtheria? |  |
| 5. What distinguishes Bacillus anthracis from other Bacillus species in terms of pathogenicity? |  |




















