General Properties of Viruses Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
| Introduction |

|
| Classification and Morphology of Viruses |

|
| Viral Replication Process |

|
| Viral Cultivation |

|
Introduction
Viruses are the tiniest infectious agents and are not made up of cells. They are different from bacteria and other simple organisms because they:
- Must live inside a host cell to survive.
- Have either DNA or RNA, but never both at the same time.
- Can be filtered because they are smaller than bacteria and can pass through filters that block bacteria.
- Cannot grow on artificial media without cells; they need to grow in animals, eggs, or tissue cultures.
- Multiply using complex methods, which is different from bacteria that reproduce by splitting in two.
Viruses do not have a proper cell structure. They do not have a cell wall, cell membrane, or any cell organelles, including ribosomes. They also do not have the enzymes needed to make proteins and nucleic acids. Additionally, they are not affected by antibiotics that target bacteria.
Classification and Morphology of Viruses
DNA Viruses: Examples include: 3P + 2H + AB
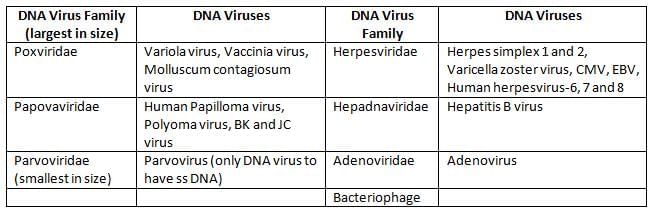
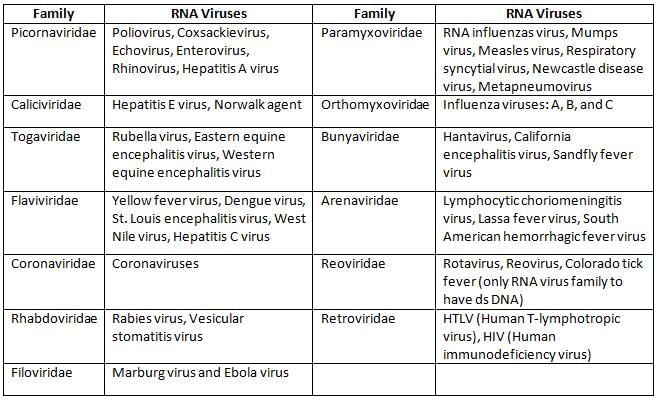
RNA Viruses
Size of Viruses
- The size of viruses is determined by:
- Ultrafiltration: This involves passing viruses through filters with different sized holes.
- Ultracentrifugation: This method uses high speeds to separate viruses based on their size and density.
- Electron microscopy: This technique allows scientists to see viruses by using a powerful microscope that uses electrons.
- Largest virus: The biggest virus is the Pox virus, which measures about 300 nm.
- Smallest virus: The smallest virus is the Parvovirus, which is only 20 nm in size.
Shape of Viruses
Most of the viruses are roughly spherical except:
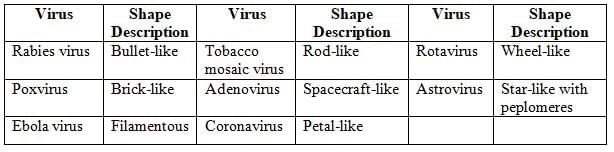
Viral Structure
Viruses consist of a nucleocapsid (nucleic acid and capsid), which is further surrounded by an envelope (in some viruses). The capsid is a protein layer made up of capsomer units.
Nucleic Acid: Viruses possess either DNA or RNA, but never both.
- Genomic size:
- Largest are retroviruses (7–11.5 kbp)
- Smallest is Hepatitis D (1.7 kb) followed by Hepatitis B (3.2 kbp)
- All the DNA viruses are double stranded, except Parvoviruses (the only ss DNA virus)
- All the RNA viruses have one copy of single stranded unsegmented RNA except:
- Reoviruses (the only double stranded RNA virus)
- Retroviruses including HIV (possess 2 copies of ss RNA)
- Segmented RNA Viruses (code-BIRA)
- Bunya virus (3 segments)
- Influenza virus (8 segments)
- Rotavirus (11 segments)
- Arenavirus (2 segments), e.g. LCM i.e. lymphocytic choriomeningitis virus
- Most RNA viruses possess positive sense RNA except: Myxoviruses, Rabies, Filoviruses, Bunyaviruses, and Arenaviruses (bear negative sense ss RNA).
Symmetry
- Icosahedron symmetry: E.g. All DNA viruses (except Pox) and most of the RNA viruses
- Helical symmetry: Few RNA viruses (Myxo, Rhabdo, Filoviridae, Bunya) (MRF-Bat)
- Pox: Complex symmetry.
Envelope
Certain viruses possess an envelope surrounding the nucleocapsid. The envelope is lipoprotein in nature.
- Made up of lipoprotein subunits called peplomere.
- The lipid part is derived from the host cell membrane and the protein part is virus-derived.
- The envelope provides chemical, physical, and biological properties to the cell.
- Enveloped viruses are ether sensitive, heat labile, and pleomorphic.
- Example: All other than nonenveloped viruses are enveloped viruses (See below).
Nonenveloped Virus
Ether resistant, heat stable, and nonpleomorphic
- DNA: Parvovirus, Adenovirus, Papovavirus (PAP)
- RNA: Picorna, Astrovirus, Reovirus, Calicivirus, and Hepatitis A and E.
Viral Replication Process
Unlike bacteria, which replicate through binary fission, viruses replicate in a more intricate manner. The process of viral replication involves six sequential steps:
Steps of Viral Replication
- Adsorption/attachment: This is the initial and highly specific step where the virus interacts with receptors on the surface of the host cell.
- Penetration: After attachment, the virus enters the host cell through one of the following methods:
- Phagocytosis (or viropexis): This occurs through receptor-mediated endocytosis.
- Membrane fusion: This method is exemplified by viruses like HIV.
- Injection of nucleic acid: This method is commonly observed in bacteriophages.
- Uncoating: During this stage, the capsid is dismantled, often due to the action of host lysozymes, leading to the release of the viral nucleic acid. The uncoating process varies among different types of viruses, including bacteriophages.
- Biosynthesis: In this step, various viral components are produced, including:
- Nucleic acid
- Capsid protein
- Enzymes
- Other regulatory proteins
- Site of Nucleic Acid Replication:
- DNA Viruses: Replication typically occurs in the nucleus. However, poxviruses are an exception, as their replication takes place in the cytoplasm.
- RNA Viruses: Replication generally occurs in the cytoplasm. Exceptions include retroviruses and ortho-myxoviruses, which replicate in the nucleus.
- Assembly: During this phase, viral nucleic acid and proteins are assembled to form new viruses, known as nucleocapsids.
- DNA Viruses: Assembly usually takes place in the nucleus. Exceptions include hepadnaviruses and poxviruses, which assemble in the cytoplasm.
- RNA Viruses: Assembly occurs in the cytoplasm.
- Maturation: This process can occur in the nucleus, cytoplasm, or cellular membranes.
- Release: New virions are released from the host cell through one of two mechanisms:
- Lysis of Host Cells: This method is common in non-enveloped viruses and bacteriophages.
- Budding: Enveloped viruses typically release by budding through the host cell membrane.
Eclipse Phase The eclipse phase refers to the period between the entry of the virus into the host cell and the first appearance of an infectious virus particle. During this phase, the virus is not detectable within the host cell. The duration of the eclipse phase is approximately: 15 to 30 minutes for bacteriophages 15 to 30 hours for most animal viruses
Classification of Viruses
- Virion: These are true viral particles that contain both capsid protein and nucleic acid.
- Prion: Prions are composed of abnormal infectious protein molecules that lack nucleic acid.
- Viroids: Viroids are small, circular, single-stranded RNA molecules that do not have a capsid. They primarily affect plants and rely on host enzymes for their replication.
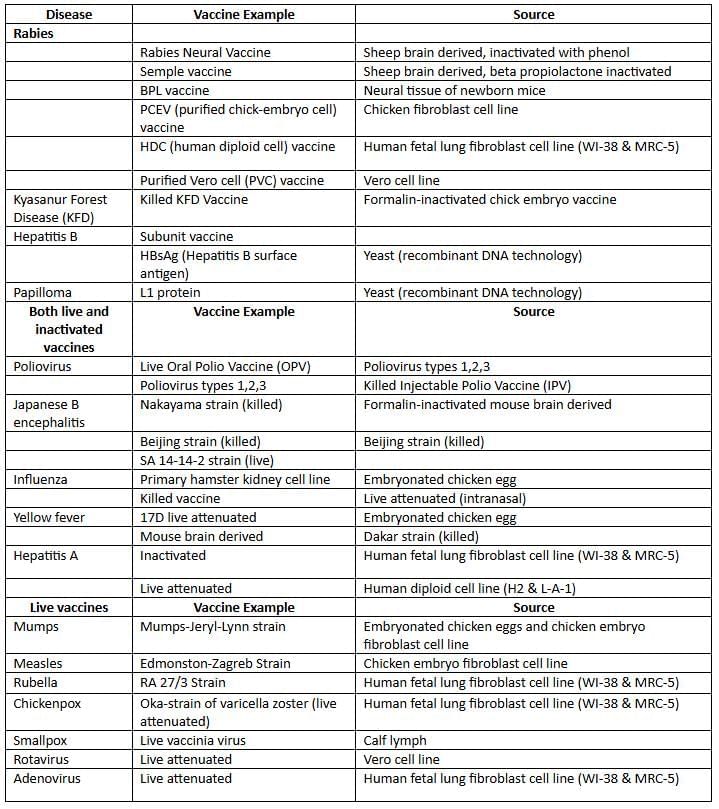
Viral Cultivation
Viruses cannot grow in artificial cell-free media; they require living hosts such as animals, eggs, or tissue cultures for their replication.
Animal Inoculation
- Suckling mice are employed to cultivate specific viruses, including Coxsackie and arboviruses.
- Coxsackie A virus induces flaccid paralysis in mice, while Coxsackie B virus causes spastic paralysis in mice.
Embryonated Egg Inoculation
- Embryonated eggs offer four potential sites for viral cultivation.
- Chorioallantoic membrane (CAM): Certain viruses, such as Vaccinia, Variola, HSV 1, and HSV 2, produce lesions known as pocks in this membrane.
- Yolk sac: Hosts arboviruses such as Japanese Encephalitis Virus (JEV), Saint Louis, and West Nile virus, as well as Rickettsia, Chlamydia, and Hemophilus ducreyi.
- Amniotic membrane: Used for influenza culture to aid in diagnosis.
- Allantoic cavity: Utilized for creating vaccines against Influenza, Yellow fever (17D), and Rabies (Flury).
Tissue Culture
- Organ culture: Involves the use of whole organs, such as tracheal rings for the cultivation of coronaviruses.
- Explant culture: Utilizes minced organs, like adenoid explants for the cultivation of Adenovirus.
- Cell Line: Tissues are thoroughly digested, and individual cells are mixed with viral growth medium before being placed in tissue culture flasks.
Types of Cell Lines
- Primary cell lines include:
- Rhesus kidney cell line
- Human amniotic cell line
- Chick embryo fibroblast
- Secondary cell lines include:
- Human fibroblast cell line
- MRC-5 cell line
- WI-38 cell line
- Continuous cell lines include:
- HeLa cell line
- HEp-2 cell line
- KB cell line
- McCoy cell line
- Vero cell line
- BHK cell line
- Detroit 6 cell line
- Chang C/I/L/K cell line
Detection of Viral Growth in Cell Line
- The cytopathic effect (CPE) is observed as changes in the structure of cells caused by viral infection in cultured cells.
- Viral Interference: The growth of a non-CPE virus can be identified by infecting the cell line with a known CPE virus. For instance, rubella, a non-CPE virus, inhibits the replication of enteroviruses, which are known to cause CPE.
- To detect viral antigens in infected cell lines, methods such as:
- Direct Immunofluorescence (IF) assay
- Immunoperoxidase staining
- Hemadsorption
- Electron microscopy is utilized to identify viral particles in infected cell lines.
- Viral gene detection is accomplished using techniques like PCR or nucleic acid probes.
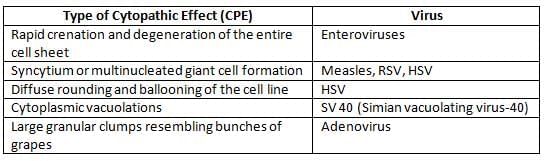
Inclusion body
They are the aggregates of virions or viral proteins and other products of viral replication by which they can be demonstrated in virus infected cells under the light microscope.
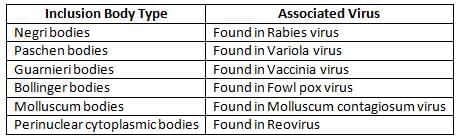

Combined Intracytoplasmic and Intranuclear Inclusion Bodies
- Observed in Measles virus and Cytomegalovirus (noted for Owl's eye appearance)
Assay of Infectivity of Viruses
- Physical Methods: Estimate the total virus count (or viral antigen or gene count) and Cannot distinguish between infectious and non-infectious virus particles.
- Real-time PCR
- Antigen detection assay
- Biological Methods: Detect the infectious virions.
- Examples include:
- Qualitative assay (end point biological assays).
- Quantitative assays (plaque assay and pock assay).
Persistent Viral Infection
Some viruses undergo a period of latency, which can be of various types:
- Latent infection with periodic exacerbations: Seen with Herpesviridae family.
- Cell transformation: Oncogenic viruses.
- Latency seen in HIV infection: Viral genome integrates with host cell chromosome, leading to clinical latency.
- Latency in slow virus infection: Have unusually long incubation periods (years).
- Persistent tolerant infection: Example is lymphocytic choriomeningitis virus infecting mice; the host is immunologically tolerant, showing no immune response, but the virus is detectable in tissues.
Transplacental Transfer of Viruses
- Teratogenic viruses (causing fetal malformation): CMV, Rubella, Herpes, Varicella, and Parvovirus B19
- Viruses that transfer through the placenta but do not cause fetal malformation: Hepatitis B and C, HIV, Coxsackie B, Measles, and Mumps
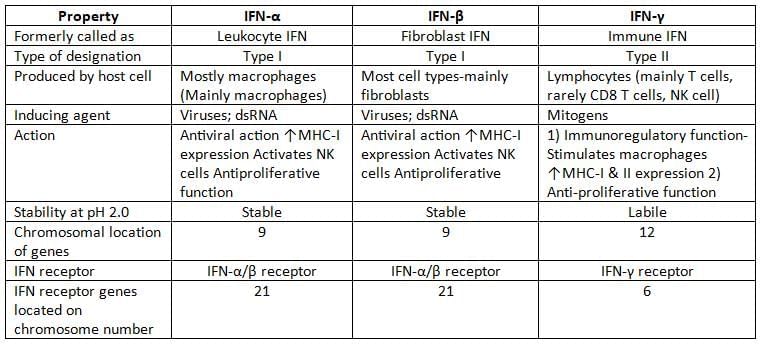
Interferons
- Interferons (IFNs) are cytokines produced by host cells in response to viral or non-viral inducers
- Classification: Interferons are classified into three groups: IFN-α, IFN-β, and IFN-γ
- Mechanism of Action:
- IFNs do not directly kill viruses but enhance the host's antiviral defences.
- They induce other host cells to produce proteins called translation inhibition proteins (TIPs), which inhibit viral protein synthesis without affecting cellular mRNA.
- IFNs are host-specific but not virus-specific.
- Inducers
- Both viral and non-viral agents can induce IFN synthesis.
- Generally, RNA viruses and avirulent viruses are strong inducers.
- Examples of potent inducers include:
- Viruses: Togaviruses, vesicular stomatitis virus, Sendai virus, and NDV (New Castle Disease virus)
- Nucleic acids (double-stranded RNA).
- Synthetic polymers (e.g., Poly I:C).
- Bacterial endotoxin.
- Resistance: IFNs are proteins and thus inactivated by proteases but not by nucleases or lipases.
- They are heat-stable and stable across a wide range of pH (except IFN-γ).
- Interferon assay is based on their biological activity; they cannot be detected serologically due to being poorly antigenic.
Application of IFN
- IFN-α is used:
- Topically: in rhinovirus infection, genital warts, and herpetic keratitis.
- Systemically: in chronic hepatitis B, C, and D infections, hairy cell leukaemia, and Kaposi’s sarcoma.
- IFN-β: Used in multiple sclerosis.
- IFN-γ: Used in chronic granulomatous disease and osteopetrosis.
|
75 docs|5 tests
|
FAQs on General Properties of Viruses Chapter Notes - Microbiology - NEET PG
| 1. What are the general properties of viruses? |  |
| 2. What distinguishes RNA viruses from DNA viruses? |  |
| 3. How is the size of viruses typically measured and what is their range? |  |
| 4. What are the different shapes of viruses and how do they affect their function? |  |
| 5. What are the methods for inoculating viruses in research and diagnostics? |  |














