HIV and Other Retroviruses Chapter Notes | Microbiology - NEET PG PDF Download
Introduction
Retroviruses
- These viruses have a special enzyme known as reverse transcriptase that helps make DNA from the viral RNA once it enters a host cell.
- Just two types are harmful to people: HIV and HTLV.
Human Immunodeficiency Virus (HIV)
Morphology
The morphology of HIV and related viruses is described in detail below:
- Envelope: HIV is spherical in shape, with a diameter ranging from 80 to 110 nanometers. The viral envelope consists of a lipid bilayer embedded with two types of proteins:
- Glycoprotein 120 (gp120): This protein forms spike-like structures on the surface of the virus, playing a crucial role in the virus's ability to infect host cells.
- Glycoprotein 41 (gp41): This protein is responsible for anchoring the glycoprotein 120 to the viral membrane and facilitates the fusion of the viral envelope with the host cell membrane.
- Nucleocapsid: The nucleocapsid of HIV has an icosahedral shape and is composed of core proteins. Inside the nucleocapsid, there is an inner core that contains:
- RNA: HIV possesses two identical strands of single-stranded positive-sense linear RNA. This RNA serves as the genetic material for the virus.
- Viral enzymes: The inner core also contains essential viral enzymes, including reverse transcriptase, integrase, and proteases. These enzymes are crucial for the viral replication process and are closely associated with HIV RNA.
HIV Genes and Antigens
HIV has three main building genes: gag, pol, and env, plus six extra genes that help control or regulate.
Structural Genes
These genes make codes for different parts of the virus.
- Gag gene: It starts as a starter protein p55, which gets cut into three proteins: p18 makes the matrix or shell antigen; p24 and p15 both make the core antigens.
- Pol gene makes codes for viral tools like reverse transcriptase, protease, and integrase.
- It begins as a starter protein that gets cut into p31, p51, and p66.
- Env gene makes codes for the outer glycoprotein gp 160, which gets cut into gp 120 and gp 41.
Non-Structural Genes
- These genes control how the virus copies itself and play a big role in how the disease happens inside the body.
- Tat acts as a gene that boosts transcription.
- Nef, which is negative factor gene, Rev for regulator of virus gene, Vif for viral infectivity factor gene.
- Vpu gene, Vpr gene, Vpx gene, and LTR sequences which are long terminal repeats.
Antigenic Variation and Diversity of HIV
HIV has a lot of antigen changes because it mutates quickly, especially in the env gene, and the reverse transcriptase enzyme makes many mistakes.
HIV Serotyping
Based on differences in the env gene, HIV has two main types: HIV-1 and HIV-2.
- HIV-1is split into three main groups: M, N, and O, with a new group called P recently discovered.
- The M group is the most common worldwide, consisting of ten subtypes known as clades (labeled A through J).
- There are also circulating recombinant forms (CRFs) which arise from mixing different subtypes. For instance, CRF01_AE is a mix of subtypes A and E.
- An infected person can have a mix of closely related viral subtypes and/or CRFs at the same time, which are referred to as quasispecies.
- While the subtypes or clades of HIV-1 do not differ in how they cause disease or behave biologically, they do vary in where they are found and how they are spread.
- Geographical distribution:
- Subtype A is mostly found in West Africa.
- Subtype B is most common in Europe, America, Japan, and Australia.
- Subtype C is the most prevalent worldwide, making up about 47% of cases. It is also the main type in South East Africa, India, and China.
- All known groups and subtypes of HIV can be found in Cameroon, which is where the virus is believed to have originated.
- Transmission:
- Asian and African subtypes (C and E) are more easily spread through heterosexual contact.
- American strains (subtype B) tend to spread more through blood and homosexual contact.
- HIV-2 consists of eight groups (A to H) and is mainly found in Africa, with some cases in other regions like India. Group A is the most common form of HIV-2.
Pathogenesis
Mode of Transmission:
- Most common way it spreads worldwide: Sexual at 75% (vaginal 60% more than anal 15%), then parent to child at 10%, injection drug use at 10%, blood transfusion at 5%, needle-stick exposure at 0.1%.
- Most common way in India: Heterosexual at 87.4%, parent to child at 5.4%, injection drug use at 1.6%, homosexual at 1.5%, blood transfusion and needle-stick together at 1%.
- Risk of spreading: Blood transfusion 90-95%, parent to child 20-40%, injection drug use 0.5-1.0%, needle-stick 0.3%, sexual intercourse (anal 0.065-0.5%, vaginal 0.05-0.1%, oral 0.005-0.1%).
Points to note:
- Spreading can happen anytime during pregnancy and breastfeeding, but highest risk during delivery.
- Risk is highest if the mother just got infected or already has AIDS.
- No proof of HIV spreading by casual touch, kissing, or bug bites.
- Viral amount is highest in blood, genital fluids, and CSF; changes in breast milk and saliva; none or very little in other body fluids or urine.
- Saliva might have blockers like fibronectin and glycoproteins that stop the virus from spreading.
Receptor Attachment and Fusion:
- Main receptor: gp120 of HIV attaches to CD4 receptor on the host cell surface. CD4 is mostly on helper T-cells but also on monocytes, macrophages, Langerhans cells, astrocytes, keratinocytes, and glial cells.
- A second coreceptor is needed for HIV to fuse by binding to gp120 and enter the host cell, for example:
- CXCR4 molecules on T-lymphocytes.
- CCR5 molecules on macrophage lineage cells.
- DC-SIGN, a lectin receptor specific to dendritic cells, can attach to HIV-1 but does not let it enter the cell. Instead, it might help dendritic cells carry HIV to lymph organs where it copies more in T-cells.
Change in CCR5 called delta 32 mutation blocks HIV from entering cells. It happens in some Europeans who are either:
- Fully resistant to HIV if they have two copies of the mutation gene, seen in 1% of Northern Europeans, especially Swedes.
- Or able to get infected but AIDS progresses slower if they have one copy, seen in 10-15% of Europeans.
- For example, someone with the homozygous delta 32 mutation might never get HIV even if exposed, showing how genetic changes can protect against viruses.
Replication
- After fusing, HIV goes through penetration and loses its coat, then HIV RNA turns into HIV DNA using RT enzyme, forming a preintegration complex with linear double-stranded DNA, gag matrix protein, accessory Vpr protein, and viral integrase, which moves into the host cell nucleus.
- Integration: The viral double-stranded DNA joins the host cell chromosome, helped by viral integrase. The joined virus is called provirus.
- Latency: In this joined state, HIV sets up a hidden infection for different times. But HIV differs from other hidden viruses because it can copy itself even when hidden and infect nearby cells.
Immunopathogenesis
The disease's natural path goes through these stages:
- Acute HIV Disease or Acute Retroviral Syndrome.
- Asymptomatic Stage.
- Persistent Generalized Lymphadenopathy (PGL).
- Symptomatic HIV Infection (or AIDS related complex, ARC).
- AIDS.
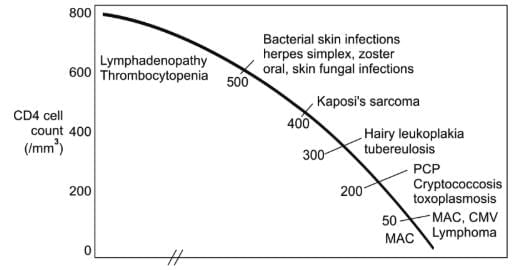 Opportunistic infections associated with HIV infection and correlation with CD4 T-cell counts
Opportunistic infections associated with HIV infection and correlation with CD4 T-cell counts
Persistent Generalized Lymphadenopathy (PGL) means enlarged lymph nodes bigger than 1 cm in two or more separate sites lasting at least 3 months. It happens in 25-30% of infected people.
Clinical Diagnosis of HIV/AIDS
Systems for classifying help track and watch the HIV spread and give doctors and patients key info on disease stage and how to manage it. Two systems are used now:
- CDC classification system (revised 1993): Splits HIV infection into nine stages based on related clinical issues and CD4 T-cell count.
- WHO clinical staging of HIV/AIDS for adults (revised 2007) based only on clinical issues. It helps in poor-resource places like India where CD4 T-cell count tests are not common.
WHO clinical staging of HIV/AIDS for adults (Revised, 2007)
- Clinical Stage 1
- Asymptomatic HIV infection.
- Persistent generalized lymphadenopathy.
- Clinical Stage 2
- Unexplained moderate weight loss less than 10%.
- Recurrent respiratory tract infection (sinusitis, tonsillitis, otitis media, pharyngitis).
- Herpes zoster.
- Angular cheilitis.
- Recurrent oral ulcers.
- Papular pruritic eruptions.
- Seborrheic dermatitis.
- Fungal nail infection.
- Clinical Stage 3
- Unexplained severe weight loss more than 10%.
- Unexplained chronic diarrhea more than 1 month.
- Unexplained persistent fever 1 month.
- Oral candidiasis.
- Oral hairy leukoplakia.
- Pulmonary tuberculosis.
- Severe bacterial infection.
- Acute necrotizing ulcerative stomatitis, gingivitis, and periodontitis.
- Unexplained anemia.
- Clinical Stage 4
HIV Wasting syndrome (Slim disease): Marked by deep weight loss more than 10%, chronic diarrhea more than 1 month, prolonged unexplained fever 1 month. - Bacterial opportunistic infections:
- Recurrent severe bacterial infections.
- Extrapulmonary tuberculosis.
- Disseminated nontubercular mycobacterial infection.
- Recurrent septicemia (including nontyphoidal salmonellosis).
- Viral opportunistic infections:
- Chronic HSV infection.
- Progressive multifocal leukoencephalopathy.
- CMV (retinitis, or other organ infection excluding liver, spleen, and lymph node).
- Fungal opportunistic infections:
- Pneumocystis jirovecii pneumonia.
- Esophageal candidiasis.
- Extrapulmonary cryptococcosis (meningitis).
- Disseminated mycoses (histoplasmosis and coccidioidomycoses).
- Parasitic opportunistic infections:
- Toxoplasma encephalitis.
- Chronic intestinal isosporiasis more than 1 month.
- Atypical disseminated leishmaniasis.
- Chronic intestinal cryptosporidiosis more than 1 month.
- Neoplasia:
- Kaposi’s sarcoma.
- Invasive cervical cancer.
- Lymphoma (cerebral, B-cell and non-Hodgkin).
- Other conditions (direct HIV induced):
- HIV encephalopathy.
- Symptomatic HIV associated nephropathy or cardiomyopathy.
- Neoplasia in HIV: Kaposi's sarcoma, Invasive cervical cancer, Lymphoma (cerebral, B cell and non-Hodgkin).
Epidemiology of HIV/AIDS
Global Situation of HIV/AIDS
- Prevalence: By 2013, around 35 million people had HIV with a world adult rate of 0.8%.
- Sub-Saharan Africa is hit hardest, with almost one in 20 adults having HIV and making up nearly 71% of people with HIV globally.
HIV/AIDS Situation in India
- By late 2015, adult HIV rate in India was 0.26%.
- Number of people living with HIV/AIDS over 21.1 lakh.
- Andhra Pradesh (undivided) was worst hit state then Maharashtra and Karnataka for number of people with HIV.
- But for rate per 100 people, Northeast states like Nagaland, Mizoram, and Manipur are worst.
- HIV prevalence: Global adult rate 0.8%, India 0.26%, Number of PLHA in India 21.17 lakh.
- Worst affected State in India: For PLHA and total HIV cases Andhra Pradesh then Maharashtra and Karnataka, Prevalence highest in Northeast states (Nagaland, Mizoram and Manipur).
Laboratory Diagnosis
Tests for detecting HIV infection
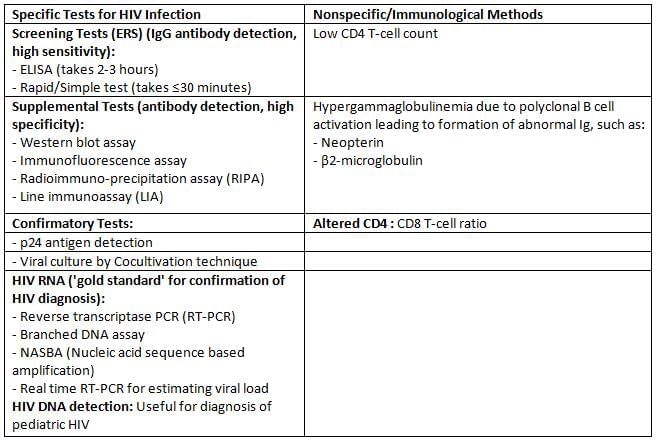
NACO Strategy for HIV Diagnosis
For the resource poor countries, it is impracticable to confirm the result of HIV screening tests by PCR or western blot as these assays are expensive and available only at limited centers.
NACO (National AIDS Control Organisation, India) has formulated a strategic plan for HIV diagnosis.
- Depending on the situation/condition, for which the test is done, the positive result of the first screening test should be either considered as such or confirmed by another one or two screening tests.
- The first screening test should be highly sensitive, whereas the second and third screening tests should have high specificity.
- The three screening tests should use different principles or different antigens. The same kit should not be used again.
- Supplemental or confirmatory tests should be used only when the screening test(s) result equivocal/intermediate.
There are four NACO strategic plans/algorithms:
- Strategy I: It is done for blood donors in blood banks. Only one test is done.
- Strategy IIa: It is done for seroprevalence or epidemiological purpose. Two tests are done.
- Strategy IIb: Purpose: It is followed for the diagnosis of HIV/AIDS in symptomatic patients. Two tests are done.
- Strategy III: It is done for the diagnosis of asymptomatic HIV patients, antenatal screening and screening of patients awaiting surgeries. Three tests are done.
Prognosis/Monitoring of HIV
Various tools available for monitoring the response to antiretroviral therapy include:
- CD4 T-cell count: Most commonly used
- HIV RNA load: Most consistent and best tool at present
- p24 antigen detection
- Neopterin and β2 macroglobulin level
Note: Antibody levels are inconsistent during late stage due to immune collapse; hence not reliable for prognosis.
Diagnosis of Pediatric HIV
- The routine screening methods (ELISA or rapid/simple tests) detect IgG antibodies.
- They cannot differentiate between baby’s IgG or maternally transferred IgG, hence cannot be used for the diagnosis of pediatric HIV.
- As all maternal antibodies would disappear by 18 months; IgG assays can only be performed after 18 months of birth.
The recommended methods for diagnosis of pediatric HIV include:
- HIV DNA detection: Most recommended
- HIV RNA detection
- p24 antigen detection
- IgG ELISA: Only after 18 months of age.
Diagnosis of HIV in Window Period
Definition: Window period refers to the initial time interval between the exposure and appearance of detectable levels of antibodies in the serum.
- The antibodies appear in blood within 2–8 weeks after infection but usually become detectable after 3 to 12 weeks with the assays available presently. It can be as low as 22 days; when third generation antibody detection kits with high sensitivity are used.
- p24 antigen detection (30% sensitive): detects by 1–2 weeks (average 16th day)
- HIV RNA detection (PCR) is the best method, detects earliest by 12th day.
Treatment: Antiretroviral Therapy (ART)
Indication to Start ART
- NACO guidelines suggest starting ART based on CD4 T-cell count and WHO clinical staging.
- For patients in Clinical Stage I and II: Begin ART if CD4 T-cell count is less than 500 cells/mm3.
- For patients in Clinical Stage III and IV: Start ART regardless of the CD4 T-cell count.
- For patients with both HIV and TB:Initiate ART without considering the CD4 T-cell count or the type of tuberculosis.
- Begin antitubercular drugs first and then start ART after two months, once the TB medication is tolerated.
- If CD4 count is below 50, both ART and ATT may be started at the same time.
- For patients with HIV and HBV/HCV co-infection who show signs of severe chronic liver disease: Start ART regardless of the CD4 count.
- For pregnant women who are HIV positive: Begin ART no matter the CD4 count.
- For patients with HIV nephropathy: Start ART.
- For patients co-infected with HIV and Visceral Leishmaniasis: Initiate ART without regard to CD4 count.
Antiretroviral drugs
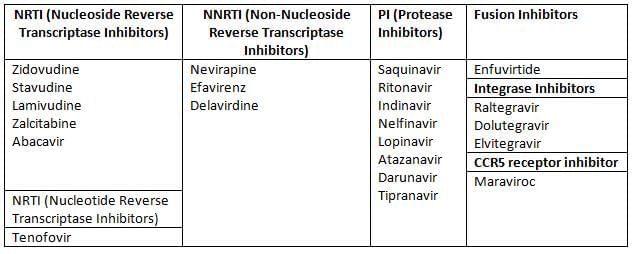
Principles for Selecting the First-line Regimen
- Highly Active Antiretroviral Therapy (HAART) is the use of a combination of at least three antiretroviral (ARV) medications.
- The main goal of HAART is to achieve maximum suppression of the HIV virus and halt the progression of HIV disease.
- Using a single drug, known as monotherapy, is not recommended and should be avoided.
Problems Pertaining to Use of ART
- Negative side effects of treatments
- High cost of Antiretroviral Therapies (ARTs)
- Limited options for treatment
- There is a risk of developing drug resistance, which can lead to the spread of a resistant virus
- IRIS: This stands for Immune Reconstitution Inflammatory Syndrome. It can happen in some people with AIDS after they start ART. As the amount of virus in the body goes down, the immune system starts to get better. However, it can react strongly to an infection that was already present, causing a severe inflammatory response. This can make the symptoms of the infection even worse.
NACO Guidelines for Postexposure Prophylaxis (PEP)
TLE Regimen
- A single tablet that includes Tenofovir (TDF) 300 mg, Lamivudine (3TC) 300 mg, and Efavirenz (EFV) 600 mg should be taken once a day for 4 weeks.
- It is best to start this treatment within 2 hours after exposure, and it must be started no later than 72 hours after exposure.
- This treatment is meant for healthcare workers (HCW) who have been exposed to:
- Source positive for HIV (either low risk/asymptomatic or high risk/symptomatic)
- Any type of exposure (whether mild, moderate, or severe)
- This treatment is also applicable when the source is unknown.
Human T-cell Lymphotropic Virus (HTLV)
Key Members: The two important members are HTLV-I and HTLV-II. Only HTLV-I is known to cause disease, as described below.
- Transmission:
- From mother to child through breast milk (this is the most common way).
- Through sexual contact between men.
- Via infected blood.
- Target Cells: This virus mainly infects CD4 T-cells, but it can also affect CD8 T-cells, dendritic cells, and B-cells.
- Target Receptor: The virus's gp protein attaches to the host cell receptor called GLUT1 (which stands for Human Glucose Transporter Protein-1).
- Tax Gene: The Tax gene of HTLV-I works as a transactivator and is responsible for the virus's ability to cause cancer.
- Distribution: HTLV-I is commonly found in certain areas of Japan (with about 10% of the population infected) and in the Caribbean basin of Africa.
- Genotypes: There are 7 genotypes of this virus; type-A is the most common, while the others are mainly found in central Africa.
- Clinical Manifestations: HTLV-I can lead to various health issues, including:
- Adult T-cell leukemia/lymphoma
- Cutaneous T-cell lymphoma
- Tropical spastic paraparesis
- Autoimmune conditions such as inflammatory diseases, uveitis, and arthropathies.
|
75 docs|5 tests
|
FAQs on HIV and Other Retroviruses Chapter Notes - Microbiology - NEET PG
| 1. What is the morphology of HIV and how does it differ from other retroviruses? |  |
| 2. What are the key genes and antigens of HIV? |  |
| 3. How does antigenic variation occur in HIV and what impact does it have on treatment and vaccine development? |  |
| 4. What is the role of non-structural genes in HIV? |  |
| 5. What are the clinical diagnosis methods for HIV/AIDS? |  |




















