Hepatitis Viruses Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
| Introduction |

|
| Hepatitis A Virus |

|
| Hepatitis B Virus |

|
| Hepatitis C Virus |

|
| Hepatitis D Virus |

|
| Hepatitis E Virus |

|
| Hepatitis G Virus |

|
Introduction
Hepatitis viruses form a captivating and diverse family of pathogens that zero in on the liver, sparking inflammation and leading to symptoms like fever, nausea, vomiting, and jaundice.
- All these viruses target the liver specifically.
- They lead to sudden swelling in the liver.
- This swelling causes the same kind of tissue damage in the liver.
- They result in similar health problems like high temperature, feeling sick, throwing up, and yellowing of skin and eyes.
- Hepatitis viruses are grouped into six kinds: HAV, HBV, HCV, HDV, HEV, and HGV.
- Other viruses that can sometimes lead to liver swelling include yellow fever virus, CMV, EBV, HSV, rubella virus, and enteroviruses.
Features of hepatitis viruses
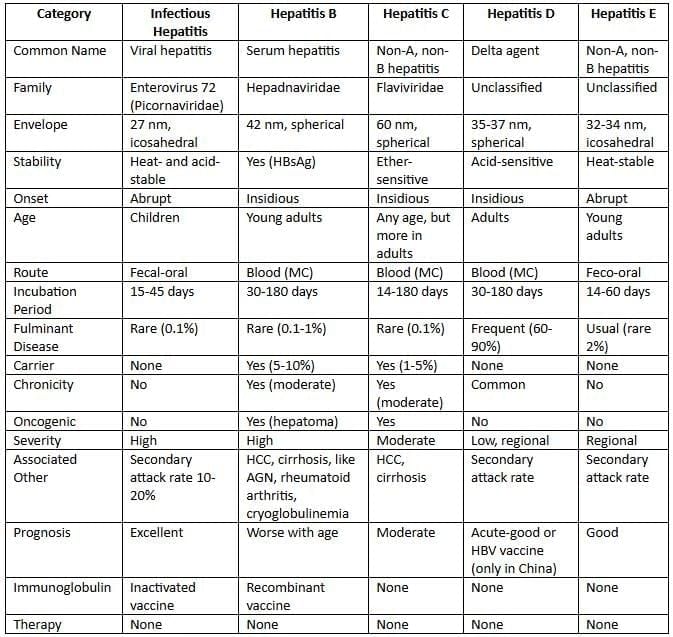
Hepatitis A Virus
- Hepatitis A virus is part of the Picornaviridae family, known as enterovirus 72 or in the Hepatovirus genus.
- It measures 27 to 32 nm and has a shape with 20 sides.
- It holds a straight single-strand RNA.
Epidemiology
- Mode of transmission
- Hepatitis A virus spreads mainly through stool-to-mouth path.
- Seldom through intimate acts like mouth-to-private parts or through blood routes.
- People who can host: Only humans serve as hosts for this virus.
- Age group affected:
- Kids and teens from 5 to 14 years are most often hit, with most cases showing no clear signs (80 to 95 percent).
- In places with better cleanliness, cases drop, and infection moves to older people.
- Grown-ups show yellowing more often (75 to 90 percent) and face higher death risk than kids.
- Cases without yellowing versus with yellowing: In kids, ratio is around 12 to 1, and in grown-ups, 1 to 3.
- Factors that increase risk: Bad personal cleanliness and too many people living close.
- Time of year: Happens all year, peaks after heavy rain and start of cold season.
- Virus release: Virus comes out in stool from 2 weeks before to 2 weeks after yellowing starts.
Laboratory Diagnosis
- Testing for anti-HAV antibodies:
- IgM type antibodies show up in the sharp phase, reach highest about 2 weeks after liver enzyme rise, and fade in 3 to 6 months.
- IgG appears a week after IgM, stays for many years, showing old infection or healing.
- Finding HAV bits using special electron microscope: HAV shows in stool from minus 2 to plus 2 weeks of yellowing.
- Finding HAV markers in stool via ELISA from minus 2 to plus 2 weeks of yellowing.
- Growing the virus: HAV is the only liver virus where growing in lab is tried, though hard, using monkey cell types.
- General signs: Higher liver enzymes and blood yellow pigment levels.
Vaccines
- Formaldehyde inactivated vaccine: This vaccine is made using human fetal lung fibroblast cell lines and is administered through intramuscular (IM) injection.
- Live attenuated vaccine: This type of vaccine utilizes the H2 and L-A-1 strains, which are prepared using human diploid cell lines from China.
- Both vaccines: They are known to be highly immunogenic, meaning they effectively stimulate the immune system and provide long-lasting immunity.
HAV-Ig
- It helps prevent after contact for close people like family or daycare folks with hepatitis A or for travelers.
- Give within 2 weeks of contact, protects for 1 to 2 months.
Hepatitis B Virus
Hepatitis B virus is the most common type of hepatitis virus.
- HBV is unique among hepatitis viruses because it is the only one that is a DNA virus.
- This virus was first identified by Baruch Blumberg in 1963.
- HBV is part of a group known as the Hepadnaviridae family.
Morphology
Using electron microscope on blood from infected people shows three shape types.
1. Round forms: Most seen, 22 nm size, fully made of HBsAg.
2. Tube or thread-like forms: 200 nm long, also fully made of HBsAg.
3. Full form or Dane particles: Less seen, 42 nm round viruses, made of.
- Outer cover: HBsAg, also called hepatitis B surface marker or cover marker or Australian marker.
- Inner 27 nm core: Has core marker HBcAg and pre-core marker HBeAg and DNA builder enzyme.
Viral Genome
- S gene has three parts: S, pre-S1, and pre-S2, they make surface marker HBsAg.
- C gene: Has pre-C and C parts, make two core proteins.
- Pre-C part makes HBeAg.
- C part makes HBcAg.
- X gene makes HBxAg.
- It might help in cancer by linking to p53.
- HBxAg and its fighter are high in people with bad long-term liver swelling and liver tumor.
- P gene is biggest, makes builder P protein with three enzyme actions: i DNA builder action, ii Reverse copy action, iii RNase H action.
Typing of HBV
- Serotypes: HBV split into four main kinds adr, adw, ayr, ayw based on fighter spots on cover protein HBsAg.
- adw main in Europe, Australia, America.
- In India adr common in south and east, ayw in west and north.
- Genotypes: Eight kinds A to H.
- Genotypes A and D common in India.
Hepatitis B Virus Mutants
Pre-core Mutants:
- There is a problem in the pre-core region of the C gene that causes these patients to be unable to produce HBeAg.
- Geographical distribution: This issue has been found in countries around the Mediterranean and in parts of Europe.
- Patients with this condition may receive a late diagnosis and often experience severe cases of chronic hepatitis, which can lead to cirrhosis.
- Markers: These patients do not have HBeAg, but other viral markers are still present.
Escape Mutants:
- There are changes in the S gene that lead to changes in HBsAg (often in an antigen).
- These changes can create challenges for hepatitis B vaccination strategies and for diagnosing the disease.
- These mutations are found in:
- Infants born to HBeAg positive mothers
- Liver transplant recipients
- A small number of individuals receiving both active and passive immunization
YMDD Mutation:
- Patients infected with HBV who are undergoing lamivudine therapy might develop resistance to the medication.
- This resistance is due to a mutation in the YMDD locus of the HBV reverse transcriptase region of the polymerase gene.
Transmission
HBV spreads through many ways.
- Blood route: In growing nations, most via blood transfer and needle pokes.
- Risk of getting HBV after needle poke is about 30 percent, versus 3 percent for HCV and 0.3 percent for HIV.
- As tiny as 0.00001 ml blood can spread HBV.
- Intimate spread common in advanced nations, like same-gender males.
- From mom to baby: Especially in China and SE Asia.
- Can happen anytime: in womb, at birth max risk, or nursing.
- Risk highest if mom has HBeAg.
- Direct touch with sick open skin spots, like impetigo, mainly in kids.
Epidemiology
Hepatitis B virus hits all over the world.
- Source of spread: Only humans, who can be sick or carriers.
- Carriers temporary hold virus weeks to months or long-term over 6 months.
- Carriers grouped into:
- Basic carriers: Low spread, send virus slowly, have low HBsAg, no HBeAg.
- Super carriers: Very spreading, send virus well, have high HBsAg, plus HBeAg, DNA builder, HBV DNA.
- Commonness: Based on HBsAg carrier numbers, three patterns.
- Type 1 low under 2 percent in Sri Lanka, Nepal.
- Type 2 medium 2 to 8 percent in India, Bhutan, Indonesia, Maldives.
- Type 3 high over 8 percent in Bangladesh, DPR Korea.
- India status: India has second biggest HBV load after China.
- India has medium HBV commonness, south Indians higher carriers.
- HBV second top cause of sudden virus liver swelling after HEV in India.
- Time of spreading: Infectious while HBsAg in blood, from waiting time a month before yellowing up to months after, sometimes years for long carriers.
- Stop spreading when HBsAg gone, replaced by anti-HBs fighter.
- Max spreading when HBeAg high in blood.
- HBV and HIV together:
- About 10 percent of all HIV people worldwide have HBV too.
- HBV doesn't change HIV path, but HIV boosts risk of HBV scarring and liver tumor.
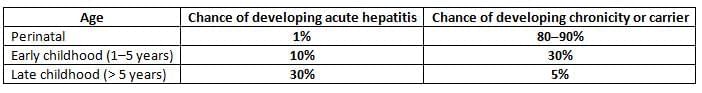
- Age:Result of HBV depends on age.
- After HBV hit.
- Chance of sudden liver swelling rises with age.
- Chance of long-term or carrier drops with age.
Laboratory Diagnosis of HBV
- Sure finding of HBV relies on blood tests for virus signs.
- It doesn't grow in usual lab setups.
Hepatitis B Surface Antigen (HBsAg):
HBsAg first sign to rise after hit, appears 1 to 12 weeks.
- Shows in waiting time, 2 to 6 weeks before lab and health signs of liver swelling.
- HBsAg present means start of spreading.
- Stays high all through sudden liver swelling.
- Becomes unseen 1 to 2 months after yellowing start, replaced by HBsAb showing healing.
- Or seldom stays over 6 months in long-term liver swelling and carrier.
- HBsAg used to track spread of hepatitis B, like to find how common it is.
Hepatitis B Pre-core Antigen (HBeAg) and HBV DNA:
They appear at the same time or shortly after the detection of HBsAg in the blood.
- They serve as indicators of:
- Active viral replication
- High viral infectivity (meaning they can easily spread the infection to others)
- These markers can be found in:
- Acute active hepatitis
- Chronic active hepatitis
- A carrier state where HBV is actively reproducing and highly infectious (these carriers are referred to as super carriers).
- However, they cannot distinguish between these different phases of infection.
- Their presence simply shows that the virus is actively multiplying.
Hepatitis B Core Antigen (HBcAg):
- HBcAg hidden due to HBsAg cover around it.
- Also not released, so not found in blood.
- But can find HBcAg in liver cells using glow light microscope.
Anti-HBc IgM (Hepatitis B Core Antibody):
- Anti-HBc IgM first fighter to rise after hit.
- Appears 1 to 2 weeks after HBsAg, lasts 3 to 6 months.
- Its presence means sudden hepatitis B hit.
- Often the only sign, sometimes with anti-HBc IgG, in gap between anti-HBs appear and HBsAg gone.
Anti-HBc IgG (Hepatitis B Core Antibody):
- Anti-HBc IgG shows up during the late acute phase of infection and stays positive for a long time, regardless of the patient's condition.
- This condition can lead to several outcomes, including:
- Chronic stage: where HBsAg remains in the body, accompanied by symptoms and high liver enzyme levels.
- Carrier state: where HBsAg is still present, but the person shows no symptoms.
- Recovery: indicated by the presence of Anti-HBs antibodies.
- Anti-HBc IgG can also act as a marker to help understand the spread of HBV infection.
Anti-HBe (Hepatitis B Precore Antibody):
- Anti-HBe fighters appear after HBeAg cleared.
- Its presence means less virus copying and lower spreading.
Anti-HBs (Hepatitis B Surface Antibody):
- Appears after HBsAg cleared, stays high forever.
- Its presence means healing, protection, and no spreading, meaning transmission stops.
- Only sign of shot protection.
Interpretation of HBV sero-markers
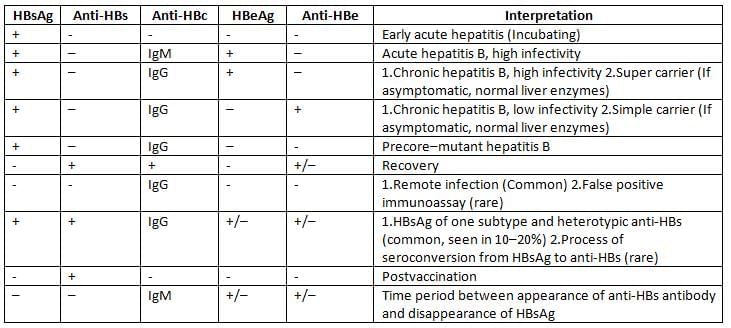
Treatment
- In sudden hepatitis B in healthy grown-ups, healing in 99 percent, so no virus-fighting drugs needed.
- Specific virus drugs used in bad sudden or bad long-term liver swelling.
- Pegylated interferon.
- Nucleoside or nucleotide like: Lamivudine, adefovir, entecavir, telbivudine, tenofovir.
Prophylaxis
Active Immunization (Hepatitis B Vaccine)
- Hepatitis B Vaccine is a type of recombinant subunit vaccine.
- The vaccine uses the surface antigen (HBsAg), which is made in Baker’s yeast using DNA recombinant technology by inserting the S gene into the yeast chromosome.
- Administration Route: Given through an intramuscular injection into the deltoid muscle (in infants, it is given in the anterolateral thigh).
- Dosage:
- 10-20 µg per dose (children under 10 years receive half the dose).
- Vaccination Schedule:
- Adults: Three doses are administered at 0, 1, and 6 months.
- National Immunization Schedule: Doses are given at 6, 10, and 14 weeks, along with the DPT vaccine. An additional dose may be given at birth in areas where the prevalence of HBV is greater than 8%.
- Minimum Interval: There should be at least 4 weeks between doses.
- Protection Marker: Individuals are considered protected if their anti-HBsAg antibody level is greater than 10 IU/ml.
- However, about 5-10% of vaccinated individuals may either not respond (non-responders) or respond slowly (low responders).
- Protection can last for approximately 15 years or more.
- Booster Doses: Required after 5 years, especially for high-risk individuals if the antibody level drops below 10 IU/ml.
Passive Immunization (Hepatitis B Immunoglobulin or HBIG)
- Indications: Used when immediate protection is needed.
- For those who have been exposed to HBsAg positive blood, such as surgeons, nurses, and lab workers.
- For sexual contacts of individuals with acute hepatitis B.
- For newborns of mothers who are hepatitis B carriers.
- For post-liver transplant patients who require protection against HBV infection.
- If there is an accidental exposure, HBIG should be administered as soon as possible (ideally within 6 hours, but no later than 48 hours).
- Recommended Dose: 0.05-0.07 ml/kg of body weight, with two doses given 30 days apart.
- HBIG provides short-term passive protection lasting about 100 days.
Combined Immunization
- For infants born to mothers infected with HBV, a combined immunization with HBIG and the vaccine is recommended.
- A single injection of 0.5 ml of HBIG is given to the newborn immediately after birth, followed by a full course of the vaccine at a different site (the first dose should be given within 12 hours of birth).
- Post-Exposure Prophylaxis Guidelines:
- If the exposed person has been vaccinated and has a protective antibody level (greater than 10 IU/ml), no further treatment is required.
- If the vaccinated person’s antibody level is not protective (less than 10 IU/ml):
- HBIG should be given immediately.
- A single vaccine dose should be administered within 7 days of exposure.
- If the exposed person is not vaccinated, both HBIG and a full course of the vaccine (3 doses) are necessary.
Hepatitis C Virus
- Hepatitis C virus often causes after-blood-transfer liver swelling in growing nations.
- HCV under Flaviviridae family, Hepacivirus genus.
- Round, 60 nm, covered virus, has positive single RNA.
Genetic Diversity of HCV
HCV shows variety in RNA due to fast changes in virus.
- Genotypes: HCV into 6 kinds or groups, differ 25 to 35 percent in RNA order.
- Subtypes: Genotypes into 100 subtypes, differ 15 to 25 percent in RNA base order.
- In one patient, subtypes as close virus group called quasispecies.
- E2 cover protein most changing area of HCV, prone to changes, leads to long-term hit, shot failure.
- HCV genotypes not differ in illness badness but in spread pattern.
- Genotype 1 most common, worldwide.
- In India, genotypes 1 and 3 more common.
- Genotypes differ in drug response.
- People with genotype 1b respond bad to therapy than others.
Transmission
- HCV spreads by blood most common, from mom lower risk 6 percent than HBV 20 percent, and intimate.
- HCV not spread via milk, food, or casual touch like hugs or kisses.
Clinical Manifestations
The incubation period for HCV infection lasts about 15 to 160 days, with an average of 50 days.
After being infected with HCV:
- Approximately 20% of individuals will experience acute hepatitis.
- Around 75% to 80% will go on to develop a chronic disease, which includes:
- 60% to 70% will develop chronic hepatitis.
- 5% to 20% may develop cirrhosis.
- 1% to 5% could develop hepatocellular carcinoma, which is responsible for 25% of all liver cancer cases.
- There are also extrahepatic manifestations due to the accumulation of immune complexes in different areas of the body, leading to:
- Mixed cryoglobulinemia
- Glomerulonephritis
- Arthritis and joint pain
Laboratory Diagnosis
- Serum antibody detection:
- Anti-HCV antibodies typically show up around 8-9 weeks after being exposed.
- These antibodies can be found in over 95% of chronic cases.
- In cases of acute hepatitis, the presence of antibodies can vary.
- Third-generation ELISAs are the most commonly used tests today.
- They use antigens from the core, NS3, NS4, and NS5 regions to find anti-HCV antibodies:
- Acute diagnosis: Detects Anti-HCV antibodies such as C33c, C22-3, and NS5.
- Chronic diagnosis: Detects Anti-HCV antibodies like C100-3, C33c, C22-3, and NS5.
- They use antigens from the core, NS3, NS4, and NS5 regions to find anti-HCV antibodies:
- HCV RNA detectionis considered the most sensitive and reliable test:
- It can be detected even before liver enzymes and HCV antibodies increase.
- This test is useful for predicting how well treatment will work, but it does not reliably indicate the severity of the disease.
Treatment
- Best plan is mixed therapy with pegylated interferon plus ribavirin.
- Start in 2 to 3 months after illness start, continue 24 weeks.
Predictors of Treatment Response
- Genotypes: Individuals with HCV genotype 1b have the most unfavorable outcome compared to all other genotypes.
- Viral RNA load: A higher viral load (greater than 800,000 IU/mL) indicates a poorer prognosis.
- Interleukin 28B is a major trigger for the release of interferon-α.
- The specific form of IL28B known as the CC genotype leads to a stronger release of IFN-α.
- Caucasians and African Americans do not have the CC genotype, which is why they tend to have a worse treatment response compared to Asians.
- Conditions like insulin resistance and obesity reduce the likelihood of responding well to HCV therapy.
Hepatitis D Virus
Hepatitis D is a type of virus that cannot grow on its own. It relies on another virus, Hepatitis B, to survive.
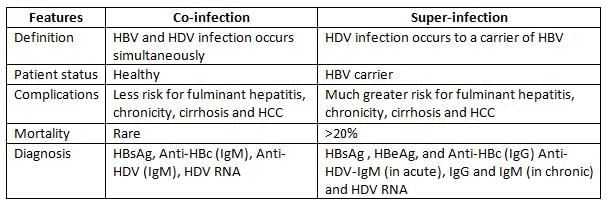
- There are two ways that Hepatitis D virus (HDV) can be present with Hepatitis B virus (HBV):
- Co-infection: This occurs when a person is infected with both viruses at the same time.
- Super-infection: This happens when someone who already has Hepatitis B gets infected with Hepatitis D.
- Morphology:
- Hepatitis D does not fit into any specific taxonomic group, but it is similar to viroids.
- Its size is about 35 nm.
- The virus is made up of:
- Circular, negative-sense single-stranded RNA (ssRNA).
- A protein coat made of a single protein known as Hepatitis D antigen (HDAg).
- A surrounding envelope made from proteins derived from Hepatitis B surface antigen (HBsAg), which is why it is referred to as a defective virus.
- Transmission: The most common way to spread Hepatitis D is through the parenteral route, which means it can be transmitted through blood. Other ways include:
- Sexual contact.
- Vertical transmission: This refers to passing the virus from mother to child during childbirt
Hepatitis E Virus
Hepatitis E virus (HEV) leads to a type of hepatitis that is mainly spread through contaminated food and water, particularly affecting young adults. This virus often causes outbreaks in developing nations.
- Although HEV is similar to caliciviruses, it actually belongs to the Hepevirus genus in the Hepeviridae family.
- Genotypes: HEV has one serotype, but there are five different genotypes identified.
- Only four of these genotypes have been found in humans.
- Genotypes 1 and 2 are more severe and only found in humans.
- Genotypes 3 and 4 can be found in both humans and other animals. These are less severe and often lead to subclinical infections.
- Fulminant hepatitis can occur in 1-2% of cases, but pregnant women have a higher risk of about 20% of developing this severe form.
Epidemiology
- Transmission: The virus spreads through fecal-oral routes, mainly from sewage pollution in drinking water or food.
- There are no chronic infections or carriers of HEV.
- Major outbreaks have been reported in India, Asia, Africa, and Central America, where HEV is the leading cause of acute hepatitis.
- HEV is unique among hepatitis viruses because it has an animal reservoir, with genotypes 3 and 4 being transmitted from animals to humans.
- In India, HEV infections account for a significant portion, about 30-60% of hepatitis cases.
- Although HEV is similar to Hepatitis A virus (HAV), there are key differences:
- The secondary attack rate (how often the virus spreads from infected individuals to others) is low at 1-2% for HEV, compared to 10-20% for HAV.
- HEV primarily affects young adults aged 20-40 years, whereas HAV tends to affect children.
Laboratory Diagnosis
- HEV RNA can be found in stool and serum even before symptoms appear, which can be detected using reverse transcriptase PCR. HEV particles can also be seen through electron microscopy.
Hepatitis G Virus
Hepatitis G virus (HGV), also known as GB virus C, was identified in 1995.
- It is related to the Hepatitis C virus and belongs to the Flaviviridae family under the Pegivirus genus.
- HGV spreads through contaminated blood or blood products, as well as through sexual contact.
- The virus replicates in the bone marrow and spleen, but it has not been linked to any specific human disease.
- HIV co-infection: HGV often coexists with HIV in about 35% of HIV-infected individuals, and this co-infection surprisingly helps patients live longer.
|
75 docs|5 tests
|
FAQs on Hepatitis Viruses Chapter Notes - Microbiology - NEET PG
| 1. What are the main types of hepatitis viruses and how do they differ in terms of transmission? |  |
| 2. How is laboratory diagnosis of hepatitis viruses typically conducted? |  |
| 3. Are there vaccines available for hepatitis viruses, and how effective are they? |  |
| 4. What is the significance of HAV-Ig in diagnosing Hepatitis A infection? |  |
| 5. What are the implications of Hepatitis B Virus (HBV) mutants in clinical settings? |  |














